Abstract
Although nitric oxide synthase (NOS) is widely considered as the major source of NO in biological cells and tissues, direct evidence demonstrating NO formation from the purified enzyme has been lacking. It was recently reported that NOS does not synthesize NO, but rather generates nitroxyl anion (NO−) that is subsequently converted to NO by superoxide dismutase (SOD). To determine if NOS synthesizes NO, electron paramagnetic resonance (EPR) spectroscopy was applied to directly measure NO formation from purified neuronal NOS. In the presence of the NO trap Fe2+-N-methyl-d-glucamine dithiocarbamate, NO gives rise to characteristic EPR signals with g = 2.04 and aN = 12.7 G, whereas NO− is undetectable. In the presence of l-arginine (l-Arg) and cofactors, NOS generated prominent NO signals. This NO generation did not require SOD, and it was blocked by the specific NOS inhibitor N-nitro-l-arginine methyl ester. Isotope-labeling experiments with l-[15N]Arg further demonstrated that NOS-catalyzed NO arose from the guanidino nitrogen of l-Arg. Measurement of the time course of NO formation demonstrated that it paralleled that of l-citrulline. The conditions used in the prior study were shown to result in potent superoxide generation, and this may explain the failure to measure NO formation in the absence of SOD. These experiments provide unequivocal evidence that NOS does directly synthesize NO from l-Arg.
Keywords: electron paramagnetic resonance, spin trapping, l-arginine, l-citrulline, superoxide
NO, a gaseous free radical, has been demonstrated to possess a variety of biological functions, including regulation of vascular tone, platelet aggregation, leukocyte adhesion, neuronal signal transmission, and immune host defense (1). Abnormalities of NO production have been hypothesized to be of central importance in the pathogenesis of many disease processes (2). In biological systems, NO is generally proposed to arise from the guanidino group of l-arginine (l-Arg) in an NADPH-dependent reaction catalyzed by a family of NO synthases (NOSs) (3). Three distinct isoforms of NOS have been cloned including neuronal NOS (nNOS, type I), inducible NOS (type II), and endothelial NOS (type III) (4). Although the three isoforms are derived from separate genes, they are believed to be similar in structure and function, with l-Arg, oxygen, and NADPH as substrates and requiring FAD, FMN, calmodulin, and tetrahydrobiopterin (BH4) as cofactors. The catalytic mechanism of NOS is hypothesized to involve to a five-electron transfer from NADPH to the terminal heme center, where O2 is incorporated into l-Arg to yield NO and l-citrulline (l-Cit) (5, 6).
In spite of intensive study of the structure and function of NOS over the last decade, there has been a lack of direct evidence that the enzyme actually synthesizes NO. This had been attributed to the labile nature of NO in oxygenated solutions, in which it is rapidly oxidized to nitrite (NO2−) and nitrate (NO3−) (7). NO also rapidly reacts with superoxide (•O2−) at diffusion-limited rate to form peroxynitrite (ONOO−) (8). Therefore, studies of the enzymatic function of NOS have largely relied on the indirect evidence of NO formation provided by measurements of the NO metabolites NO2− and NO3− or the coproduct l-Cit. Unfortunately, the data from indirect measurements cannot resolve whether NO is the primary product of NOS. In spite of strong evidence that NO formation from NOS occurs in biological cells and tissues, controversy has remained regarding whether the enzyme directly synthesizes NO. Recently, based on their failure to detect NO from isolated NOS, Schmidt et al. (9) proposed that NOS might primarily produce nitroxyl anion (NO−) rather than NO. They hypothesized that NO was alternatively derived from NO− in a reaction catalyzed by superoxide dismutase (SOD).
To address this controversy, we applied electronic paramagnetic resonance (EPR) spectroscopy to measure NO from purified NOS in an effort to determine whether the enzyme does directly synthesize NO. Our study demonstrates that NOS does generate NO and elucidates why prior efforts to detect NO formation may have failed.
MATERIALS AND METHODS
Materials.
The l-Arg, NADPH, FAD, BH4, calmodulin, N-nitro-l-arginine methyl ester (l-NAME), SOD, and other reagents were purchased from Sigma, unless otherwise noted. Cell culture materials were obtained from GIBCO/BRL. 2′,5′-ADP-Sepharose was the product of Pharmacia Biotech. l-[14C]Arg was purchased from DuPont/NEN. l-[15N]Arg (guanidino 15N, 99% pure) was obtained from Isotec. N-Methyl-d-glucamine dithiocarbamate (MGD) was synthesized as described (10). The N-methyl-d-glucamine and carbon disulfide required for MGD synthesis were purchased from Aldrich. S-Nitroso-N-acetylpenicillamine (SNAP) was synthesized as reported (11). Sodium trioxodinitrate (Na2N2O3, Angeli’s salt) was synthesized according to the procedure described in ref. 12.
NOS Purification.
Rat nNOS was purified from stably transfected human kidney 293 cells (13). These nNOS-transfected cells were grown in minimum essential medium with 10% heat inactivated fetal calf serum. Cells were harvested and homogenized in 50 mM Tris⋅HCl buffer (pH 7.4), containing 1 mM EGTA, 1 mM diethylenetriaminepentaacetic acid (DTPA), and 10 mM 2-mercaptoethanol (standard buffer) with 1 mM phenylmethylsulfonyl fluoride (PMSF), and 2 μM leupeptin. After centrifugation, the supernatant was loaded on a 2′,5′-ADP-Sepharose affinity column. After washing the column with 0.45 M NaCl, NOS was eluted with standard buffer containing 10 mM NADPH and concentrated by using Centricon-30 concentrators (Amicon). Excess NADPH, DTPA, and 2-mercaptoethanol was removed by repetitive washing of the enzyme followed by 40-fold concentration, with 50 mM Tris⋅HCl buffer (pH 7.4) with 1 mM PMSF and 2 μM leupeptin. Concentrated enzyme was stored in this buffer with 10% glycerol at −80°C. Protein content was assayed with Bradford reagent (Bio-Rad) by using BSA standards (14). The purity of NOS was greater than 90% as determined by SDS/PAGE with Coomassie blue staining. The enzyme preparations exhibited specific activity up to 450 nmol l-Cit/min per mg protein assayed by the conversion of l-[14C]Arg to l-[14C]Cit at 37°C as described below. For the spin trapping measurements ≈1% volume of the enzyme preparation was mixed with the assay buffer.
EPR Spectroscopy and Spin Trapping.
The Fe2+ complex of MGD [Fe2+-MGD2, (Fe–MGD)] was used to trap NO formation (15, 16). Fresh stock solutions of Fe–MGD (1:5) were prepared by adding ferrous ammonium sulfate to aqueous solutions of MGD. NO measurements were performed in 50 mM Tris⋅HCl buffer (pH 7.4) with 1 mM l-Arg, 1 mM NADPH, 0.5 mM Ca2+, 10 μg/ml calmodulin, 10 μM BH4, 25 μg/ml BSA, and 1 mM Fe–MGD. Another spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (Aldrich) was used to measure oxygen-free radicals at a final concentration of 50 mM. EPR spectra were recorded in a quartz flat cell at room temperature with a Bruker ER 300 spectrometer operating at X-band with 100 kHz modulation frequency and a TM 110 cavity, as described (17, 18). The microwave frequency and magnetic field were precisely measured by using an EIP 575 microwave frequency counter and Bruker ER 035 NMR gaussmeter. Quantitation of the observed signals was performed by comparing the double integral of the observed signal with that of known concentration NO–Fe–MGD standards prepared with either NO gas or the NO donor SNAP. To obtain rapid complete release of NO from SNAP photolysis was performed by using light irradiation with a one million candlepower light source focused as a 1-cm-diameter beam onto the EPR flat cell which was placed in the cavity with front plate removed. This photolysis resulted in rapid quantitative release of NO from SNAP or other nitrosothiols. The intensity of the EPR signals of the NO–Fe–MGD complexes derived from SNAP after photolysis were identical to those obtained by using identical concentrations of NO gas. After 5 min of photolysis complete release of NO from SNAP was observed. This photolysis approach was also used to evaluate whether nitrosothiol compounds were formed in the NOS reaction system.
l-[14C]Arg to l-[14C]Cit Conversion Assay.
NOS-catalyzed l-[14C]Arg to l-[14C]Cit conversion was monitored in a total volume 300-μl buffer containing 50 mM Tris⋅HCl (pH 7.4), 200 μM l-[14C]Arg, 1 mM NADPH, 0.5 mM Ca2+, 10 μg/ml calmodulin, 10 μM BH4, and 2 μg/ml purified NOS. The reaction was started by adding l-[14C]Arg. After incubation periods at ambient temperature, 23°C, the reactions were terminated with 3 ml ice-cold stop buffer (20 mM Hepes, pH 5.5/2 mM EDTA/2 mM EGTA). l-[14C]Cit was separated by passing reaction mixture through Dowex AG 50W-X8 (Na+ form, Bio-Rad) cation exchange columns and quantitated by liquid scintillation counting (19).
RESULTS
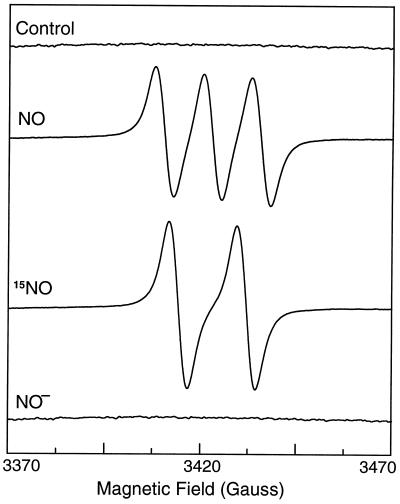
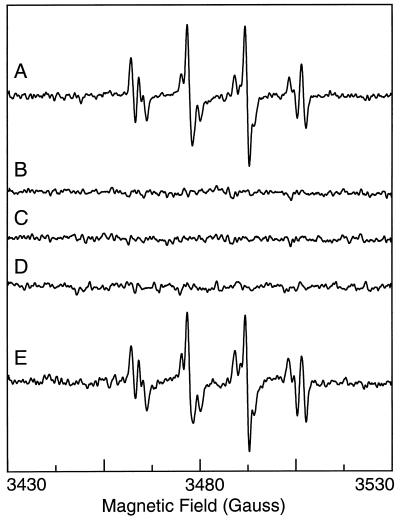
EPR measurements were performed by using the trap Fe–MGD that reacts with NO forming a stable NO–Fe–MGD complex with unique triplet EPR spectrum. As shown in the control experiment with Fe–MGD alone, no signal is seen (Fig. 1, control). However, after incubation with the NO donor SNAP (10 μM), a characteristic triplet spectrum of the NO complex was observed with a central g value of 2.04 and nitrogen hyperfine splitting of 12.7 G. Triplet splitting is observed because the natural abundance isotope of nitrogen, 14N, has a nuclear spin of one. In the presence of isotopically labeled 15NO released from 15N-SNAP (10 μM), the 15NO–Fe–MGD complex is formed which has doublet hyperfine splitting arising from the nuclear spin of 15N which has a value of one-half (16, 20). In principle only NO bound to Fe2+-MGD would be expected to give the characteristic triplet signal, and if a complex was formed with NO− it would be diamagnetic and EPR silent (7). To confirm that the Fe–MGD complexes used enable selective detection of NO, experiments were performed with addition of the NO− donor Angeli’s salt, Na2N2O3, which decomposes in aqueous buffer to form 1 equivalent of NO− and 1 equivalent of nitrite (9). No significant EPR signal was seen from Fe–MGD in the presence of 10 μM Angeli’s salt. Thus, only NO gave rise to a characteristic EPR spectrum.
Figure 1.
EPR spectra of Fe–MGD in the absence and presence of NO or NO−. Control, 1 mM Fe–MGD in 50 mM Tris⋅HCl buffer (pH 7.4); NO, Fe–MGD with the NO (14N) donor N-SNAP (10 μM); 15NO, with 10 μM 15N-SNAP; NO−, with 10 μM NO− donor Na2N2O3. In the presence of the trap Fe–MGD, natural abundance 14NO gives rise to the characteristic triplet spectrum whereas 15NO gives rise to a characteristic doublet. NO− did not produce any significant signal. Spectra were recorded at room temperature with a microwave frequency of 9.77 GHz by using 80 mW of microwave power and 3.9 G modulation amplitude.
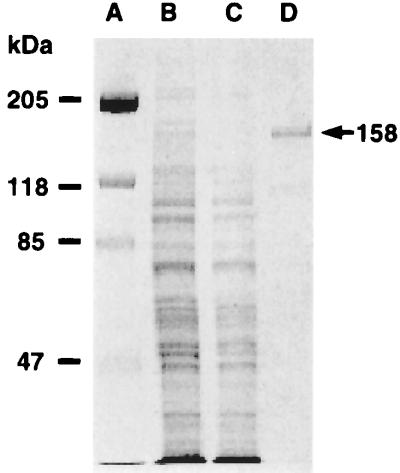
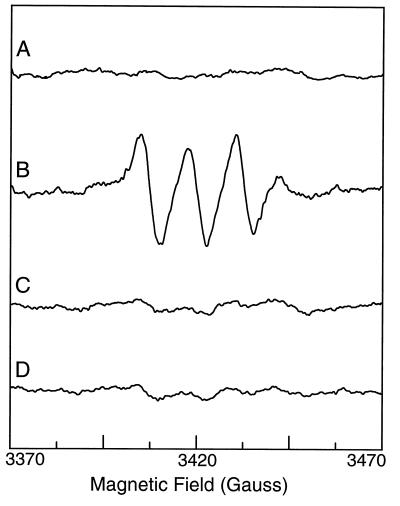
EPR measurements with Fe–MGD were then performed to measure NO generation from purified nNOS. The enzyme was purified from nNOS transfected cells and the purity was greater than 90% as determined by SDS/PAGE (Fig. 2). Although numerous bands were seen in whole cell homogenate, after purification a single 158-kDa band was present corresponding to the molecular weight of nNOS as previously reported (3). No 16-kDa or 32-kDa bands corresponding to the molecular weigh of the monomeric or dimeric forms of copper zinc SOD were present. In the absence of the enzyme, no signals were observed from solutions containing the NOS substrate l-Arg and its cofactors (Fig. 3, trace A). However, after adding purified NOS (7.5 μg/ml), prominent EPR signals were seen (Fig. 3, trace B). These signals exhibited the characteristic triplet spectrum with g = 2.04 and aN = 12.7, indicative of NO–Fe–MGD. In contrast to the prior report, SOD was not required for NO formation. NO generation was totally abolished by either excluding l-Arg from the reaction system or preincubating the enzyme with the specific NOS blocker l-NAME (Fig. 3, traces C and D), further confirming that the measured NO generation was synthesized by NOS.
Figure 2.
SDS/PAGE profile of nNOS purified from nNOS-transfected 293 cells. Lanes: A, molecular weight standards; B, crude supernatant of cell homogenate (10 μg); C, eluate from the column washed by 0.45 M NaCl (10 μg); D, NADPH eluate from 2′,5′-ADP-Sepharose column (1 μg). Proteins were separated on 7.5% polyacrylamide gels and visualized by Coomassie blue staining.
Figure 3.
NO generation from purified NOS. The reaction system consists of l-Arg (1 mM), NADPH (1 mM), Ca2+ (0.5 mM), calmodulin (10 μg/ml), and BH4 (10 μM) in 50 mM Tris⋅HCl buffer (pH 7.4). Although no signal could be detected in the reaction system without enzyme (trace A), prominent NO signal was seen after adding 7.5 μg/ml purified NOS (trace B). This NO signal could be abolished by either excluding the NOS substrate l-Arg from the reaction system (trace C) or pretreating the enzyme with the NOS blocker l-NAME (10 mM) (trace D). Representative spectra were shown from triplicate measurements.
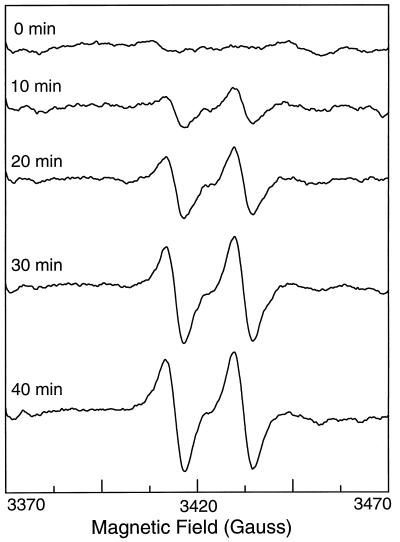
To further prove that the measured NO formation was derived from the guanidino group of l-Arg, experiments were performed by using isotopically labeled l-Arg in which both guanidino nitrogens were labeled with 15N. In the presence of this l-[15N]Arg, time-dependent formation of the characteristic 15NO–Fe–MGD spectrum was observed. This spectrum was clearly seen within the first 10 min after addition of l-[15N]Arg and it continued to increase over the next 30 min (Fig. 4).
Figure 4.
Time course of 15NO generation from NOS in the presence of l-[15N]Arg. The composition of the reaction mixture was the same as described in the legend to Fig. 2 except l-[15N]Arg was used instead of l-Arg. Spectra were continuously recorded from the beginning of the reaction until 40 min.
It has been suggested that nitrosothiols may be important intermediates in the process of NO formation or transport (7). To evaluate whether a detectable pool of nitrosothiols was formed, photolysis experiments were performed with EPR trapping of NO with Fe–MGD. After 50 min of the NOS reaction, light irradiation did not induce further NO formation. With kinetic measurements performed in the presence of light irradiation from the start of the reaction, no alterations in the magnitude or time course of the appearance of the NO signals were seen. Thus, there was no evidence of nitrosothiol formation.
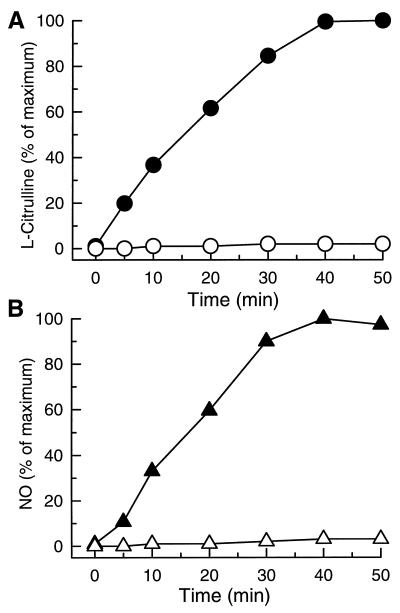
To further evaluate the kinetics and stoichiometry of NO formation from NOS relative to that of the coproduct l-Cit, parallel experiments were conducted to determine the time course of l-Cit formation by monitoring the conversion of l-[14C]Arg to l-[14C]Cit. As shown in Fig. 5, the time course of NOS-catalyzed l-Cit formation paralleled that observed for NO. The magnitude of l-Cit formation after 40-min incubation was 0.54 μmol/mg protein whereas the value obtained for NO trapped by Fe–MGD was 0.21 μmol/mg protein. In the presence of the NOS blocker l-NAME, both l-Cit formation and NO formation were abolished. Together, these experiments unambiguously prove that NOS oxidizes the guanidino nitrogen of l-Arg with the formation of NO and l-Cit.
Figure 5.
Time course of NO and l-Cit generation from NOS. l-Cit formation was detected by monitoring the conversion of l-[14C]Arg to l-[14C]Cit (A) and NO was measured by EPR with spin trap Fe–MGD (B) in parallel experiments. As shown, similar time-course curves were observed for NOS-catalyzed l-Cit (•) and NO (▴) formation. In the presence of the NOS blocker l-NAME, both l-Cit and NO formation were blocked (open symbols). Results are the average of three experiments.
In their recent study, Schmidt et al. (9) reported that NO could not be measured from purified NOS unless large amounts of SOD were present. They proposed that NOS first synthesized NO− and then SOD converted NO− to NO. However, an alternative explanation for their requirement for SOD for NO detection may be because of the existence of exogenous •O2− production. Because •O2− rapidly reacts with NO resulting in the formation of ONOO−, this could also explain their inability to measure NO formation from the enzyme. To test this hypothesis, we determined whether •O2− generation occurred in the reaction system used by the authors. With the well-characterized oxygen radical trap DMPO, prominent signals were seen in solutions containing only the substrates and cofactors used in their NO detection experiments (Fig. 6, trace A). The signals consisted of a mixture of the superoxide adduct DMPO-OOH and as well as DMPO-OH which can be formed from the degradation of DMPO-OOH (21). SOD (400 units/ml) totally abolished these signals, demonstrating that both were derived from the trapping of •O2− (Fig. 6, trace B). Because a number of cofactors had been used including FAD, NADPH, and BH4, we then sought to determine which were responsible for the •O2− generation. Neither NADPH (1 mM) nor FAD (10 μM) alone produced signals (Fig. 6, traces C and D). However, strong signals were seen in the solutions containing these two compounds together (Fig. 5, trace E). The magnitude of these signals approached that generated from the solution with all cofactors. Interestingly, whereas BH4 was previously reported to be capable of producing •O2− through autoxidation (22), addition of BH4 (10 μM) did not significantly alter the •O2− signals produced by FAD and NADPH. Thus, a potent source of •O2− existed within the reaction system used in this previous study that was mainly because of the reduction of FAD by NADPH.
Figure 6.
Oxygen radical generation from FAD and NADPH: (trace A) 50 mM Tris⋅HCl buffer (pH 7.4) containing 1 mM NADPH, 10 μM FAD, 10 μM BH4, 0.5 mM Ca2+, and 10 μg/ml calmodulin; (trace B) in the presence of SOD (400 units/ml); (trace C) 10 μM FAD; (trace D) 1 mM NADPH; and (trace E) 10 μM FAD and 1 mM NADPH. Spectra were recorded with 50 mM DMPO with a microwave frequency of 9.77 GHz, 20 mW of microwave power, and 0.5 G modulation amplitude. Each spectrum is the sum of five 1-min acquisitions.
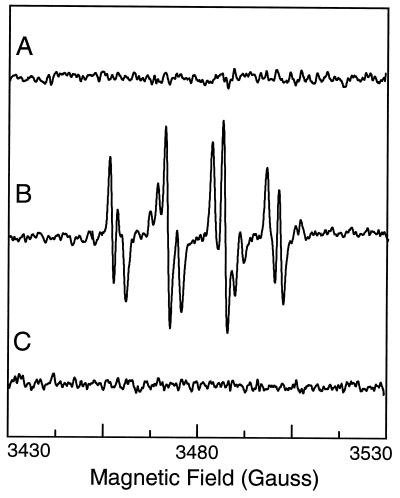
Under the conditions of enzyme, substrate, and cofactors that we used to measure NO formation from NOS, there was no detectable •O2− generation as evidenced by the absence of any EPR signal in the presence of DMPO (Fig. 7, trace A). To further demonstrate that the lack of signal was not because of SOD in our enzyme preparations, additional experiments were performed with NOS in the absence of l-Arg, conditions previously shown to induce •O2− generation from the enzyme (13, 17). In the absence of l-Arg, prominent DMPO-OOH and DMPO-OH signals were observed which were quenched by addition of exogenous SOD, 200 units/ml (Fig. 7, traces B and C). Thus under conditions that induce •O2− generation, it was detected. This confirms that SOD was not present in our enzyme preparations and that SOD is not required for NO generation from NOS.
Figure 7.
•O2− generation from purified nNOS: (trace A) 50 mM Tris-HCl buffer (pH 7.4) containing 1 mM NADPH, 10 μM BH4, 0.5 mM Ca2+, 10 μg/ml calmodulin, 1 mM l-Arg, and nNOS (7.5 μg/ml); (trace B) as in trace A but with no l-Arg; and (trace C) with addition of SOD, 200 units/ml. EPR spectra were recorded in presence of 50 mM DMPO as described for Fig. 6, except that each is the sum of ten 1-min acquisitions.
DISCUSSION
Over a decade ago a family of arginine metabolizing enzymes was discovered whose function was reported to be the synthesis of the free radical gas NO. Three specific NOS isoforms were identified: neuronal NOS (NOS1), macrophage NOS (NOS2), and endothelial NOS (NOS3). Each of these enzymes have considerable structural homology and each have been shown to exert a range of important functions in biological cells and tissues. NOS was considered to be a specific NO synthesizing enzyme which exerts important cell signaling effects through its interaction with heme enzymes such as guanylate cyclase. Early studies that used chemiluminescence or mass spectroscopy showed that l-Arg-derived NO formation occurred in endothelial cells (23, 24). In tissues and cultured cells NOS-originated NO generation was also demonstrated by NO-selective electrodes as well as EPR measurements (15–18, 25). However, direct evidence of NO formation from isolated NOS has been lacking. Because of the failure to detect NO formation from purified NOS, it has recently been suggested that NOS does not synthesize NO. It was reported that NOS actually synthesizes NO−, which can rapidly react to form nitrous oxide, N2O (9). To definitively establish that NO is the primary product of NOS, a technique is needed which can specifically detect NO and distinguish it from its other redox forms. Because only NO is paramagnetic while its redox forms NO− or nitrosonium (NO+) are diamagnetic, EPR spectroscopy provides a unique ability to specifically measure NO. NO reacts with a variety of metal complexes to form high affinity nitroso complexes, and the distinctive EPR spectra of these nitroso complexes have been applied to perform quantitative measurements of NO generation (15, 17, 20, 26). In this study we used the ferrous iron complex of MGD, Fe–MGD, to trap NO. These NO–Fe–MGD complexes exhibit a characteristic triplet EPR spectrum. Fe–MGD could also discriminate NO from NO−, as evidenced by the fact that no signal was detected from Fe–MGD in the presence of NO− formed from Angeli’s salt. Therefore, EPR measurements that used the NO trap Fe–MGD enabled definitive measurement of NO formation.
We have previously shown that NOS-catalyzed NO formation can be measured in nNOS-transfected human kidney 293 cells by using Fe–MGD (17). In this study, we performed in vitro measurements of NO generation from purified nNOS. In the presence of the substrate l-Arg and cofactors, strong NO signals were detected from NOS. NO signals could be abolished by NOS blockade, confirming that NO was generated by NOS. By using isotopically labeled l-[15N]Arg, NOS was directly demonstrated to catalyze NO formation from the guanidino nitrogen of l-Arg. Photolysis experiments did not provide evidence for significant nitrosothiol formation. The synchronous time course of NO and l-Cit formation was also in accordance with previous reports that NOS simultaneously produced NO and l-Cit (5, 6). Thus, all these lines of evidence demonstrated that NOS does synthesize NO.
Schmidt et al. (9) recently reported that SOD was required for in vitro NO detection from isolated NOS. They hypothesized that NOS primarily synthesized NO− which subsequently was converted by SOD to NO (9). Results from the present study did not support this hypothesis because SOD was not required for NO generation. Both SDS gel electrophoresis and functional measurements of •O2− generation from the enzyme, in the absence of l-Arg, confirmed that SOD was not present in our NOS preparations. The requirement for SOD in the prior experiments of Schmidt et al. (9) was probably due to the presence of large amounts of exogenous •O2− generation under their conditions of measurement. EPR spin trapping measurements demonstrated that prominent •O2− generation occurred in their reaction system that was mainly generated from the reaction of FAD and NADPH. Although FAD is often added to compensate for possible in vitro loss of FAD during enzyme purification, the concentrations used of 5–10 μM were quite high relative to the 3–50 nM concentrations of NOS in their preparations. Our data demonstrated that FAD at these concentrations consumed NADPH to generate large amounts of •O2−, which would be expected to rapidly react with NO to form ONOO−. High amounts of SOD could effectively scavenge •O2− and enable NO detection. Hence, SOD in Schmidt’s experiments may serve to simply quench •O2− rather than directly participate in NO synthesis. With exclusion of excess FAD from the reaction mixture, NO was successfully detected by EPR spin trapping. In addition, because FAD also consumes NADPH, the NADPH stoichiometry of NOS might be overestimated if extra FAD was included in the reaction.
Beyond FAD and NADPH, nNOS itself could become another source of •O2− at low levels of l-Arg (13, 27). It can be inferred from Mayer’s report (27), that NOS will switch from NO to •O2− generation when l-Arg is below 100 μM, and that at 10 μM l-Arg •O2− production would predominate. Schmidt et al. (9) used 10–100 μM l-Arg in their measurements. Under these conditions, NOS would synthesize both NO and •O2−, which would in turn react to form ONOO−. This phenomenon has been previously shown to occur in intact l-Arg-depleted cells where NOS generates both NO and •O2− leading to ONOO− formation (17). Thus, at the lower l-Arg levels NOS-catalyzed •O2− may be another reason why they required high concentrations of SOD for NO detection. In the present study, 1 mM l-Arg was used, and the presence of this substrate was shown to be sufficient to totally prevent uncoupled NOS from producing •O2−. Regarding potential •O2− generation from BH4 autoxidation, no detectable •O2− was seen with 10 μM BH4, suggesting that BH4 was not a significant source of •O2−.
Taken together, our measurements provide direct evidence demonstrating that NOS does produce NO. However, it is important to note that these data do not prove that NO is the only nitrogen oxide product formed by NOS. By comparing NO and l-Cit formation, we did not achieve a 1:1 stoichiometry. In fact, we found that the production of l-Cit was always higher than that of NO. A similar phenomenon was also reported in other studies with indirect NO measurements (28). This may be because of differences in the efficiency of the techniques used in NO and l-Cit detection. It is also possible that a fraction of the NO synthesized may be rapidly oxidized to NO2− and NO3− which are not directly detectable by EPR. In addition, NOS may also generate other reactive nitrogen species. From our data, partial formation of NO− or other nitrogen oxides cannot be excluded. Further investigation will be needed to fully characterize the mechanism of NO formation from NOS and the potential formation of other products. Nevertheless, the data reported here conclusively demonstrate that NOS does synthesize NO.
Acknowledgments
We are indebted to Dr. Alexandre Samouilov for his generous help in synthesizing reagents and valuable advice. This work was supported by National Institutes of Health Grants HL-38324 and HL-52315 (J.L.Z.). Y.X. was supported by a grant from American Heart Association Maryland Affiliate (MDFW3797).
ABBREVIATIONS
- NO−
nitroxyl anion
- NOS
nitric oxide synthase
- nNOS
neuronal NOS
- BH4
tetrahydrobiopterin
- EPR
electron paramagnetic resonance
- MGD
N-methyl-d-glucamine dithiocarbamate
- DMPO
5,5-dimethyl-1-pyrroline-N-oxide
- l-NAME
N-nitro-l-arginine methyl ester
- SOD
superoxide dismutase
- SNAP
S-nitroso-N-acetylpenicillamine
References
- 1.Moncada S, Palmer R M, Higgs E A. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Gross S S, Wolin M S. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 3.Bredt D S, Snyder S H. Annu Rev Biochem. 1991;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C, Xie Q-w. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 5.Marletta M A. J Biol Chem. 1993;268:12231–12234. [PubMed] [Google Scholar]
- 6.Griffith O W, Stuehr D J. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 7.Stamler J S, Singel D J, Loscalzo J. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 8.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt H H H W, Hofmann H, Schindler U, Shutenko Z S, Cunningham D D, Feelisch M. Proc Natl Acad Sci USA. 1996;93:14492–14497. doi: 10.1073/pnas.93.25.14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinobu L A, Jones S G, Jones M M. Acta Pharmacol Toxicol. 1984;54:189–194. doi: 10.1111/j.1600-0773.1984.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 11.Field, L., Dilts, R. V., Ravichandaran, R., Lanhert, P. G. & Carnahan, G. E. (1978) J. Chem. Soc. Chem. Commun. 249–250.
- 12.Smith P A S, Hein G E. J Am Chem Soc. 1960;82:5731–5740. [Google Scholar]
- 13.Pou S, Pou W S, Bredt D S, Snyder S H, Rosen G M. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 14.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Komarov A, Mattson D, Jones M M, Singh P K, Lai C S. Biochem Biophys Res Commun. 1993;195:1191–1198. doi: 10.1006/bbrc.1993.2170. [DOI] [PubMed] [Google Scholar]
- 16.Zweier J L, Wang P, Samouilov A, Kuppusamy P. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 17.Xia Y, Dawson V L, Dawson T M, Snyder S H, Zweier J L. Proc Natl Acad Sci USA. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweier J L, Wang P, Kuppusamy P. J Biol Chem. 1995;270:304–307. doi: 10.1074/jbc.270.1.304. [DOI] [PubMed] [Google Scholar]
- 19.Bredt D S, Snyder S H. Proc Natl Acad Sci USA. 1989;86:9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanin A F, Mordvintcev P I, Hauschildt S, Mulsch A. Biochim Biophys Acta. 1993;1177:37–42. doi: 10.1016/0167-4889(93)90154-h. [DOI] [PubMed] [Google Scholar]
- 21.Finkelstein E, Rosen G M, Rauckman E J. Mol Pharmacol. 1982;21:262–265. [PubMed] [Google Scholar]
- 22.Mayer B, Klatt P, Werner E R, Schidt K. J Biol Chem. 1995;270:655–659. doi: 10.1074/jbc.270.2.655. [DOI] [PubMed] [Google Scholar]
- 23.Palmer R M J, Ashton D S, Moncada S. Nature (London) 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 24.Palmer R M J, Ferrige A G, Moncada S. Nature (London) 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 25.Malinski T, Taha Z. Nature (London) 1992;358:676–678. doi: 10.1038/358676a0. [DOI] [PubMed] [Google Scholar]
- 26.Lancaster J R, Jr, Langehr J M, Bergonia H A, Murase N, Simmons R L, Hoffman R A. J Biol Chem. 1992;267:10994–10998. [PubMed] [Google Scholar]
- 27.Heinzel B, John M, Klatt P, Bohme E, Mayer B. Biochem J. 1992;281:627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyengar R, Stuehr D J, Marletta M A. Proc Natl Acad Sci USA. 1987;84:6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]