Abstract
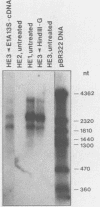
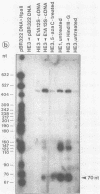
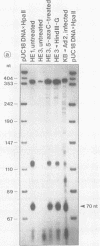
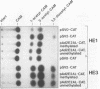
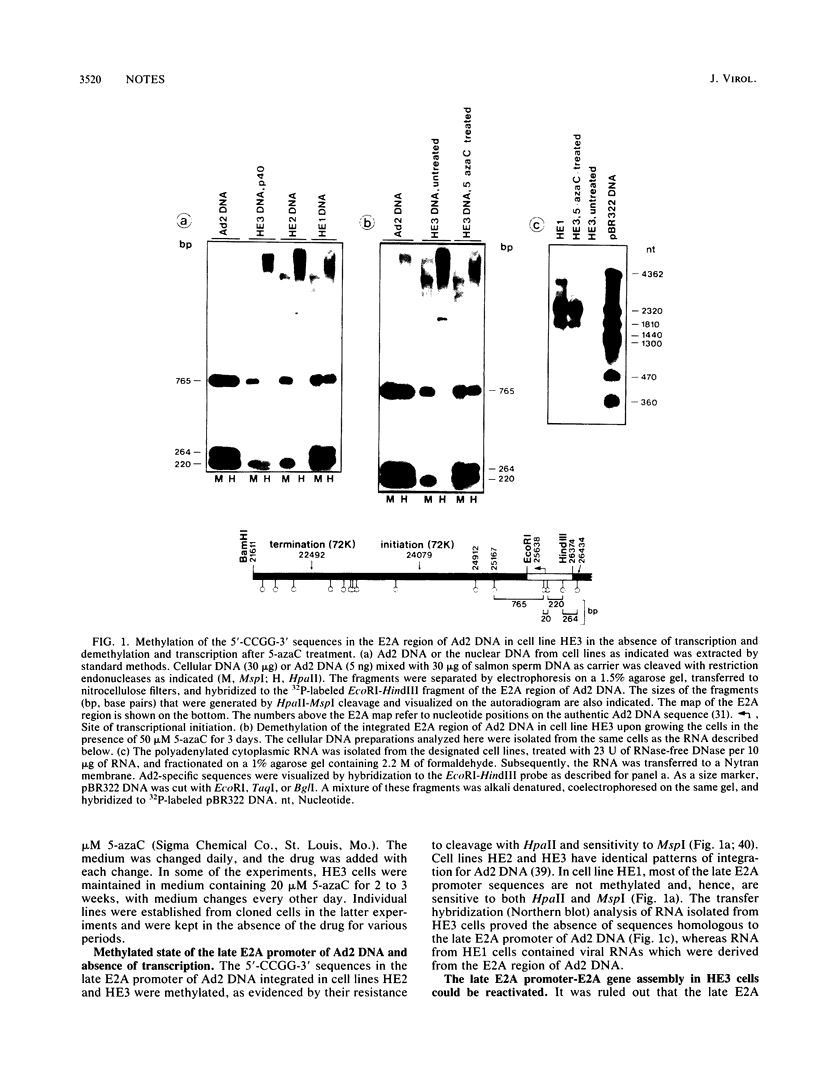
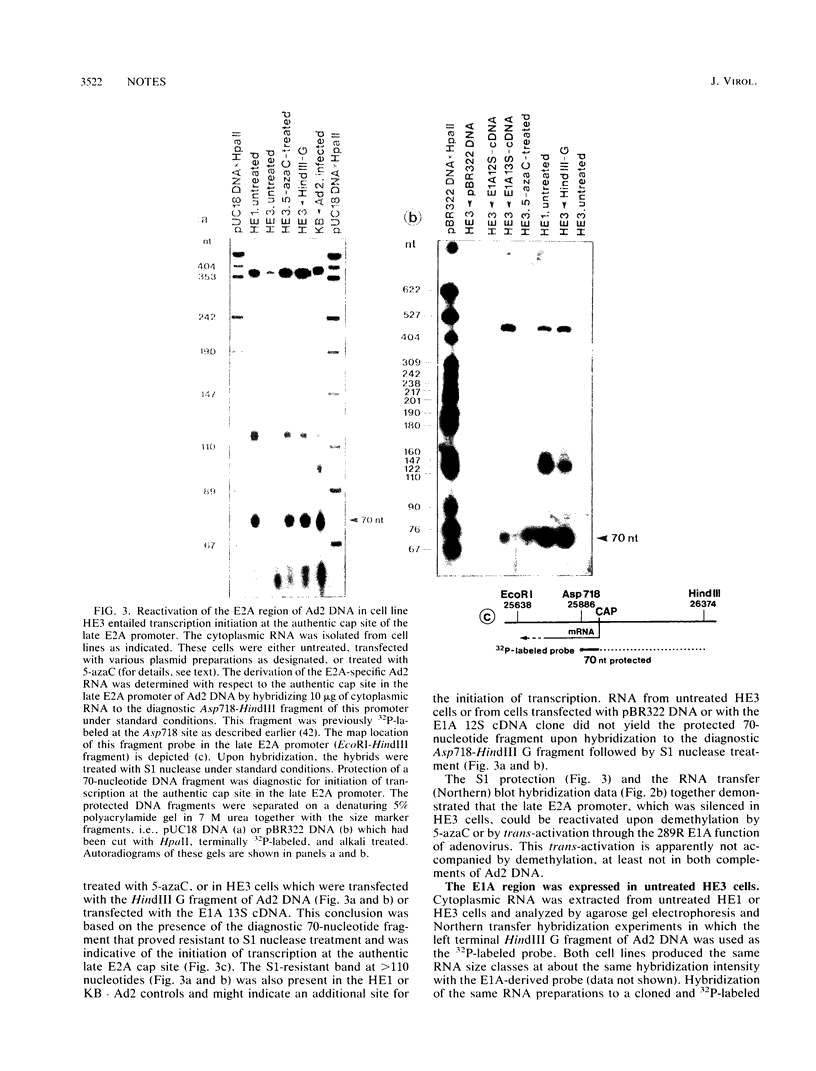
In the adenovirus type 2 (Ad2)-transformed hamster cell line HE3, the integrated late E2A promoter of Ad2 DNA is inactive, is methylated at all three 5'-CCGG-3' sequences, and can be reactivated by growing the cells in the presence of 50 microM 5-azacytidine (5-azaC). The three 5'-CCGG-3' sequences then become demethylated. Demethylation and reactivation are stable over 30 passages even after the removal of 5-azaC. The dormant late E2A promoter in cell line HE3 can also be reactivated by transfecting the cells with recombinant plasmids that carry the left terminal E1A and part of the E1B region of Ad2 DNA or the E1A 13S cDNA, but not with plasmids containing the E1A 12S cDNA. The E1A 13S cDNA encodes the 289-amino-acid trans-activating protein of Ad2. The E1A-mediated reactivation of the late E2A promoter is not accompanied by its demethylation in both DNA complements. Cell line HE3 produces constitutively E1A-encoded mRNAs and reactivates the methylated late E2A promoter-chloramphenicol acetyltransferase gene construct after transfection into HE3 cells. Constitutive levels of the endogenous E1A gene products in HE3 cells are detectable but, paradoxically, appear insufficient to reactivate the endogenous, chromosomally integrated E2A gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Cook J. L., Lewis A. M., Jr Host response to adenovirus 2-transformed hamster embryo cells. Cancer Res. 1979 May;39(5):1455–1461. [PubMed] [Google Scholar]
- Cooper D. N. Eukaryotic DNA methylation. Hum Genet. 1983;64(4):315–333. doi: 10.1007/BF00292363. [DOI] [PubMed] [Google Scholar]
- Dobrzanski P., Hoeveler A., Doerfler W. Inactivation by sequence-specific methylations of adenovirus promoters in a cell-free transcription system. J Virol. 1988 Nov;62(11):3941–3946. doi: 10.1128/jvi.62.11.3941-3946.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation--a regulatory signal in eukaryotic gene expression. J Gen Virol. 1981 Nov;57(Pt 1):1–20. doi: 10.1099/0022-1317-57-1-1. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973 Aug;54(2):536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Knebel-Mörsdorf D., Achten S., Langner K. D., Rüger R., Fleckenstein B., Doerfler W. Reactivation of the methylation-inhibited late E2A promoter of adenovirus type 2 by a strong enhancer of human cytomegalovirus. Virology. 1988 Sep;166(1):166–174. doi: 10.1016/0042-6822(88)90158-4. [DOI] [PubMed] [Google Scholar]
- Knebel D., Doerfler W. N6-methyldeoxyadenosine residues at specific sites decrease the activity of the E1A promoter of adenovirus type 12 DNA. J Mol Biol. 1986 May 20;189(2):371–375. doi: 10.1016/0022-2836(86)90518-8. [DOI] [PubMed] [Google Scholar]
- Kruczek I., Doerfler W. Expression of the chloramphenicol acetyltransferase gene in mammalian cells under the control of adenovirus type 12 promoters: effect of promoter methylation on gene expression. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7586–7590. doi: 10.1073/pnas.80.24.7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann I., Doerfler W. Loss of viral genomes from hamster tumor cells and nonrandom alterations in patterns of methylation of integrated adenovirus type 12 DNA. J Virol. 1983 Sep;47(3):631–636. doi: 10.1128/jvi.47.3.631-636.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann I., Doerfler W. Shifts in the extent and patterns of DNA methylation upon explanation and subcultivation of adenovirus type 12-induced hamster tumor cells. Virology. 1982 Apr 15;118(1):169–180. doi: 10.1016/0042-6822(82)90330-0. [DOI] [PubMed] [Google Scholar]
- Langner K. D., Vardimon L., Renz D., Doerfler W. DNA methylation of three 5' C-C-G-G 3' sites in the promoter and 5' region inactivate the E2a gene of adenovirus type 2. Proc Natl Acad Sci U S A. 1984 May;81(10):2950–2954. doi: 10.1073/pnas.81.10.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner K. D., Weyer U., Doerfler W. Trans effect of the E1 region of adenoviruses on the expression of a prokaryotic gene in mammalian cells: resistance to 5' -CCGG- 3' methylation. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1598–1602. doi: 10.1073/pnas.83.6.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U., Doerfler W. Fixation of the unmethylated or the 5'-CCGG-3' methylated adenovirus late E2A promoter-cat gene construct in the genome of hamster cells: gene expression and stability of methylation patterns. J Virol. 1987 Dec;61(12):3710–3720. doi: 10.1128/jvi.61.12.3710-3720.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Schirm S., Doerfler W. Expression of viral DNA in adenovirus type 12-transformed cells, in tumor cells, and in revertants. J Virol. 1981 Sep;39(3):694–702. doi: 10.1128/jvi.39.3.694-702.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated viral DNA sequences in hamster cells transformed by adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):565–568. doi: 10.1101/sqb.1980.044.01.058. [DOI] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Doerfler W. Patterns of integration of viral DNA in adenovirus type 2-transformed hamster cells. J Mol Biol. 1981 Apr 5;147(2):227–246. doi: 10.1016/0022-2836(81)90439-3. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucleic Acids Res. 1980 Jun 11;8(11):2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B., Langner K. D., Jüttermann R., Müller U., Zock C., Klimkait T., Doerfler W. Reactivation of the methylation-inactivated late E2A promoter of adenovirus type 2 by E1A (13 S) functions. J Mol Biol. 1988 Jul 20;202(2):255–270. doi: 10.1016/0022-2836(88)90456-1. [DOI] [PubMed] [Google Scholar]