Abstract
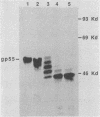
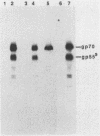
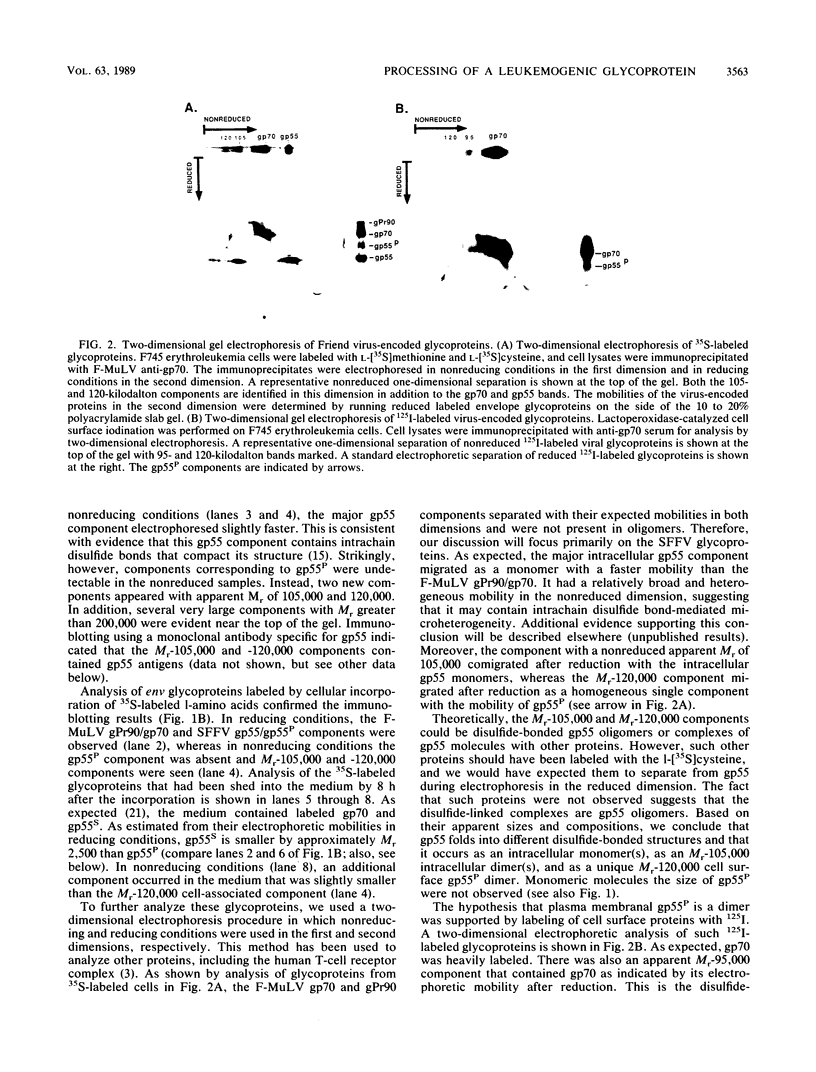
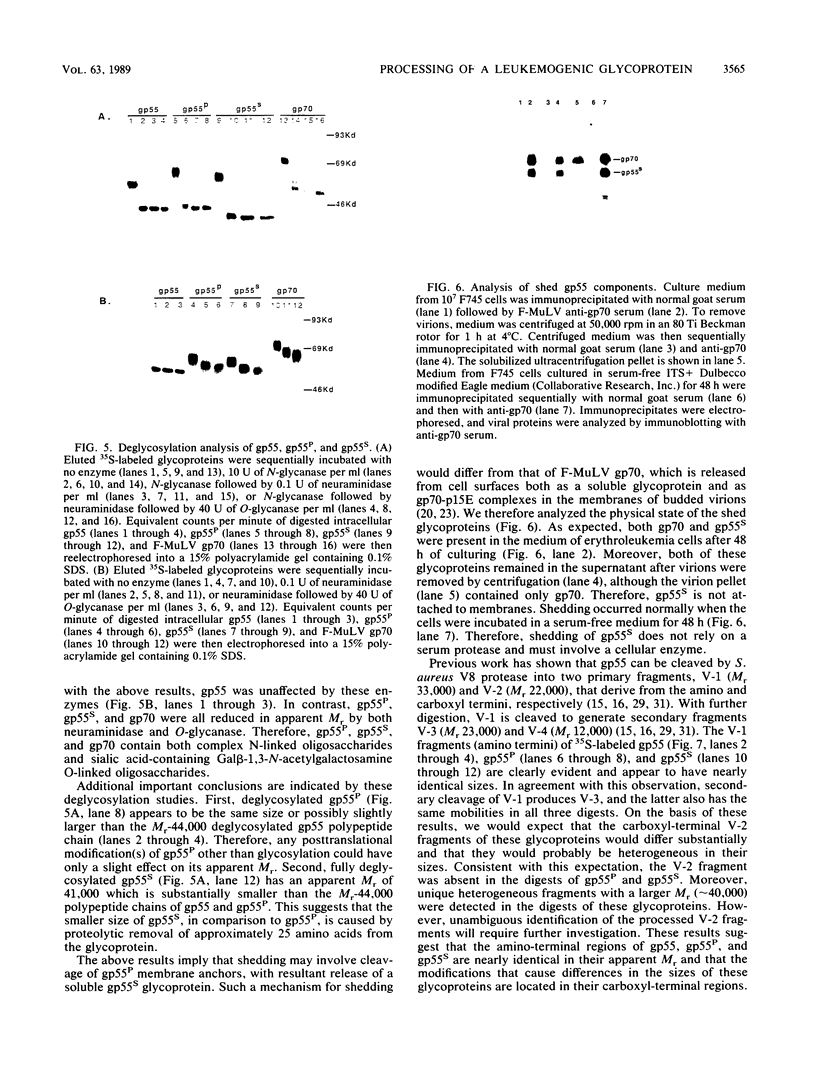
The leukemogenic glycoprotein (gp55) encoded by Friend spleen focus-forming virus is predominantly retained in the rough endoplasmic reticulum (RER). However, a small proportion (ca. 5%) is processed to form a derivative that occurs on plasma membranes and causes mitosis of infected erythroblasts. We have now found that gp55 folds heterogeneously in the RER to form components with different disulfide bonds and that this difference may determine their processing fates. RER gp55 consists predominantly of monomers with intrachain disulfide bonds. In contrast, the processed molecules are disulfide-bonded dimers. These dimers are extensively modified in transit to cell surfaces by conversion of four N-linked high-mannose oligosaccharides to complex derivatives and by attachment of a sialylated O-linked oligosaccharide. The plasma membrane dimers are then slowly shed into the medium by a mechanism that involves proteolytic cleavage of approximately 25 membrane-anchoring hydrophobic amino acids from the carboxyl termini of the glycoproteins. Consequently, shed molecules have shorter polypeptide chains than cell-associated gp55. We conclude that gp55 folds into different disulfide-bonded components that do not substantially isomerize, and that only one specific dimer is competent for export from the RER. Mitogenic activity of gp55 could be caused by the cell surface dimers, by the shed derivative, or by the carboxyl-terminal hydrophobic anchors that remain in the membranes after the shedding reaction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma H., Katori A., Obata M., Sagata N., Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Boswell B. A., Kabat D. Molecular cloning of biologically active Rauscher spleen focus-forming virus and the sequences of its env gene and long terminal repeat. J Virol. 1984 Sep;51(3):695–705. doi: 10.1128/jvi.51.3.695-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Complete nucleotide sequence of an infectious clone of Friend spleen focus-forming provirus: gp55 is an envelope fusion glycoprotein. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5037–5041. doi: 10.1073/pnas.80.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S., Ruta M., Murray M. J., Kabat D. Glycoprotein encoded by the Friend spleen focus-forming virus. J Virol. 1979 May;30(2):564–575. doi: 10.1128/jvi.30.2.564-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Troxler D. Polycythemia- and anemia-inducing erythroleukemia viruses exhibit differential erythroid transforming effects in vitro. Cell. 1980 Dec;22(3):693–699. doi: 10.1016/0092-8674(80)90545-0. [DOI] [PubMed] [Google Scholar]
- Kabat D., Ruta M., Murray M. J., Polonoff E. Immunoselection of mutants deficient in cell surface glycoproteins encoded by murine erythroleukemia viruses. Proc Natl Acad Sci U S A. 1980 Jan;77(1):57–61. doi: 10.1073/pnas.77.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Machida C., Kabat D. Role of a membrane glycoprotein in Friend virus erythroleukemia: nucleotide sequences of nonleukemogenic mutant and spontaneous revertant viruses. J Virol. 1986 Feb;57(2):534–538. doi: 10.1128/jvi.57.2.534-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Saltiel A. R. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988 Jan 15;239(4837):268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Boswell B. A., Kabat D. Role of a membrane glycoprotein in Friend virus-induced erythroleukemia: studies of mutant and revertant viruses. Virology. 1985 Jul 15;144(1):158–172. doi: 10.1016/0042-6822(85)90314-9. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Kabat D. Reduced leukemogenicity caused by mutations in the membrane glycoprotein gene of Rauscher spleen focus-forming virus. J Virol. 1984 Feb;49(2):394–402. doi: 10.1128/jvi.49.2.394-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson T. S., Bamberger M. J., Lane M. D. Post-translational changes in tertiary and quaternary structure of the insulin proreceptor. Correlation with acquisition of function. J Biol Chem. 1988 May 25;263(15):7342–7351. [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987 Sep 10;329(6135):172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Pinter A., Fleissner E. The presence of disulfide-linked gp70-p15(E) complexes in AKR murine leukemia virus. Virology. 1977 Dec;83(2):417–422. doi: 10.1016/0042-6822(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. O-linked glycosylation of retroviral envelope gene products. J Virol. 1988 Mar;62(3):1016–1021. doi: 10.1128/jvi.62.3.1016-1021.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Honnen W. J. The mature form of the Friend spleen focus-forming virus envelope protein, gp65, is efficiently secreted from cells. Virology. 1985 Jun;143(2):646–650. doi: 10.1016/0042-6822(85)90406-4. [DOI] [PubMed] [Google Scholar]
- Pinter A., Lieman-Hurwitz J., Fleissner E. The nature of the association between the murine leukemia virus envelope proteins. Virology. 1978 Dec;91(2):345–351. doi: 10.1016/0042-6822(78)90382-3. [DOI] [PubMed] [Google Scholar]
- Polonoff E., Machida C. A., Kabat D. Glycosylation and intracellular transport of membrane glycoproteins encoded by murine leukemia viruses. Inhibition by amino acid analogues and by tunicamycin. J Biol Chem. 1982 Dec 10;257(23):14023–14028. [PubMed] [Google Scholar]
- Ruddon R. W., Krzesicki R. F., Norton S. E., Beebe J. S., Peters B. P., Perini F. Detection of a glycosylated, incompletely folded form of chorionic gonadotropin beta subunit that is a precursor of hormone assembly in trophoblastic cells. J Biol Chem. 1987 Sep 15;262(26):12533–12540. [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Biological and biochemical differences between variants of spleen focus-forming virus can be localized to a region containing the 3' end of the envelope gene. J Virol. 1985 Dec;56(3):717–722. doi: 10.1128/jvi.56.3.717-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Spleen focus-forming virus: relationship of an altered envelope gene to the development of a rapid erythroleukemia. Curr Top Microbiol Immunol. 1984;112:21–44. doi: 10.1007/978-3-642-69677-0_2. [DOI] [PubMed] [Google Scholar]
- Ruta M., Bestwick R., Machida C., Kabat D. Loss of leukemogenicity caused by mutations in the membrane glycoprotein structural gene of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4704–4708. doi: 10.1073/pnas.80.15.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Clarke S., Boswell B., Kabat D. Heterogeneous metabolism and subcellular localization of a potentially leukemogenic membrane glycoprotein encoded by Friend erythroleukemia virus. Isolation of viral and cellular processing mutants. J Biol Chem. 1982 Jan 10;257(1):126–134. [PubMed] [Google Scholar]
- Ruta M., Kabat D. Plasma membrane glycoproteins encoded by cloned Rauscher and Friend spleen focus-forming viruses. J Virol. 1980 Sep;35(3):844–853. doi: 10.1128/jvi.35.3.844-853.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Buss J. E. The covalent modification of eukaryotic proteins with lipid. J Cell Biol. 1987 Jun;104(6):1449–1453. doi: 10.1083/jcb.104.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro C., Gliniak B., Kabat D. A tagged helper-free Friend virus causes clonal erythroblast immortality by specific proviral integration in the cellular genome. J Virol. 1988 Nov;62(11):4129–4135. doi: 10.1128/jvi.62.11.4129-4135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas R. V., Compans R. W. Glycosylation and intracellular transport of spleen focus-forming virus glycoproteins. Virology. 1983 Mar;125(2):274–286. doi: 10.1016/0042-6822(83)90201-5. [DOI] [PubMed] [Google Scholar]
- Srinivas R. V., Compans R. W. Membrane association and defective transport of spleen focus-forming virus glycoproteins. J Biol Chem. 1983 Dec 10;258(23):14718–14724. [PubMed] [Google Scholar]
- Srinivas R. V., Kilpatrick D. R., Compans R. W. Intracellular transport and leukemogenicity of spleen focus-forming virus envelope glycoproteins with altered transmembrane domains. J Virol. 1987 Dec;61(12):4007–4011. doi: 10.1128/jvi.61.12.4007-4011.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Lowy D., Howk R., Young H., Scolnick E. M. Friend strain of spleen focus-forming virus is a recombinant between ecotropic murine type C virus and the env gene region of xenotropic type C virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4671–4675. doi: 10.1073/pnas.74.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Koller R., Ruscetti S. Monoclonal antibody to spleen focus-forming virus-encoded gp52 provides a probe for the amino-terminal region of retroviral envelope proteins that confers dual tropism and xenotropism. J Virol. 1982 Aug;43(2):472–481. doi: 10.1128/jvi.43.2.472-481.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Scolnick E., Ruscetti S. Envelope gene of the Friend spleen focus-forming virus: deletion and insertions in 3' gp70/p15E-encoding region have resulted in unique features in the primary structure of its protein product. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4718–4722. doi: 10.1073/pnas.80.15.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]