Abstract
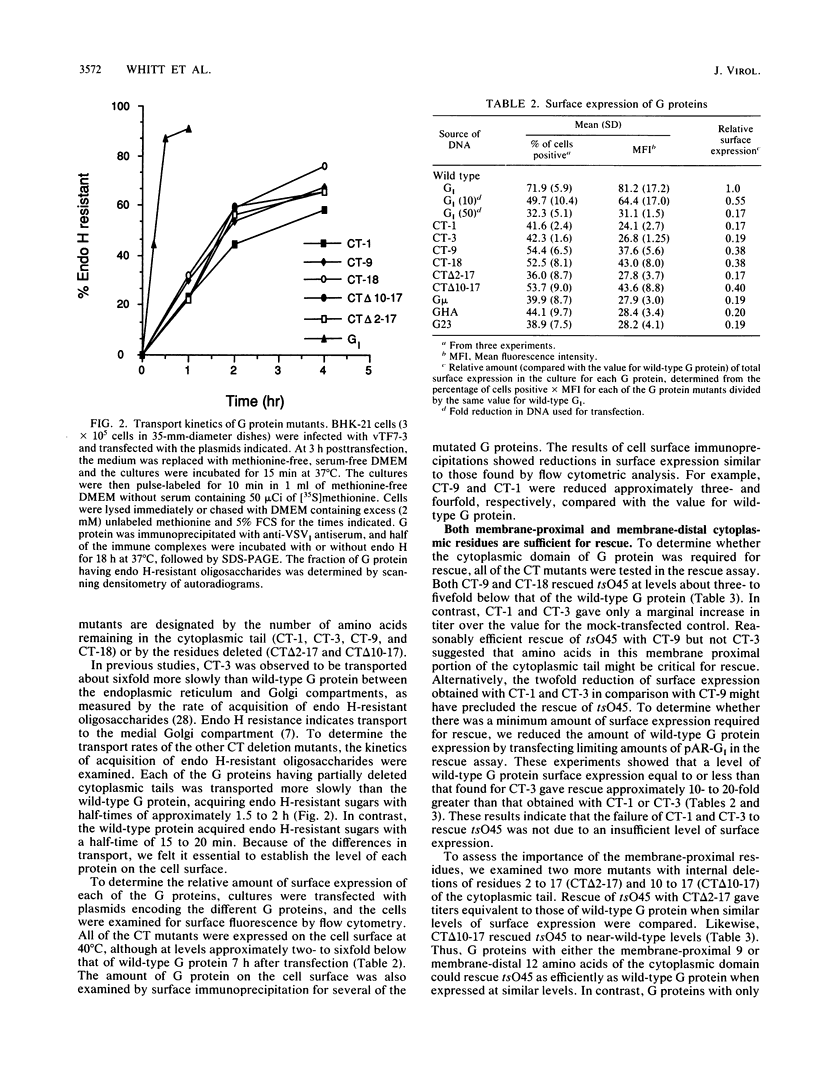
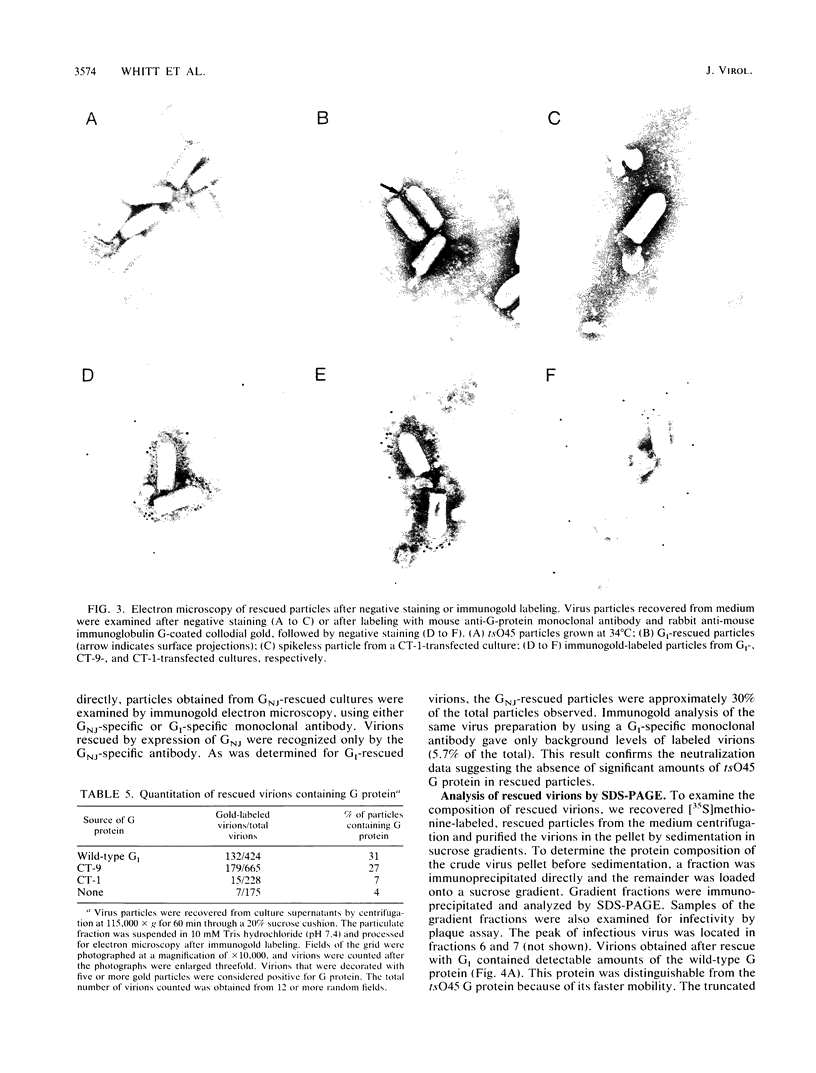
We have used transient expression of the wild-type vesicular stomatitis virus (VSV) glycoprotein (G protein) from cloned cDNA to rescue a temperature-sensitive G protein mutant of VSV in cells at the nonpermissive temperature. Using cDNAs encoding G proteins with deletions in the normal 29-amino-acid cytoplasmic domain, we determined that the presence of either the membrane-proximal 9 amino acids or the membrane-distal 12 amino acids was sufficient for rescue of the temperature-sensitive mutant. G proteins with cytoplasmic domains derived from other cellular or viral G proteins did not rescue the mutant, nor did G proteins with one or three amino acids of the normal cytoplasmic domain. Rescue correlated directly with the ability of the G proteins to be incorporated into virus particles. This was shown by analysis of radiolabeled particles separated on sucrose gradients as well as by electron microscopy of rescued virus after immunogold labeling. Quantitation of surface expression showed that all of the mutated G proteins were expressed less efficiently on the cell surface than was wild-type G protein. However, we were able to correct for differences in rescue efficiency resulting from differences in the level of surface expression by reducing wild-type G protein expression to levels equivalent to those observed for the mutated G proteins. Our results provide evidence that at least a portion of the cytoplasmic domain is required for efficient assembly of the VSV G protein into virions during virus budding.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Fusco P. J. The M protein of vesicular stomatitis virus associates specifically with the basolateral membranes of polarized epithelial cells independently of the G protein. J Cell Biol. 1988 Nov;107(5):1707–1715. doi: 10.1083/jcb.107.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Keller D. S., Helenius A., Balch W. E. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J Cell Biol. 1987 Nov;105(5):1957–1969. doi: 10.1083/jcb.105.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Ruusala A., Machamer C., Helenius J., Helenius A., Rose J. K. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988 Jul;107(1):89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Rothman J. E. Compartmental organization of the Golgi stack. Cell. 1985 Aug;42(1):13–21. doi: 10.1016/s0092-8674(85)80097-0. [DOI] [PubMed] [Google Scholar]
- Flamand A. Etude génétique du virus de la stomatite vésiculaire: classement de mutants thermosensibles spontanés en groupes de complémentation. J Gen Virol. 1970 Sep;8(3):187–195. doi: 10.1099/0022-1317-8-3-187. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Smith A., Bergmann J. E., Rose J. K. Isolation of stable mouse cell lines that express cell surface and secreted forms of the vesicular stomatitis virus glycoprotein. J Cell Biol. 1983 Nov;97(5 Pt 1):1381–1388. doi: 10.1083/jcb.97.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985 May;54(2):374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. Nucleotide sequence of a cDNA clone encoding the entire glycoprotein from the New Jersey serotype of vesicular stomatitis virus. J Virol. 1983 Apr;46(1):162–169. doi: 10.1128/jvi.46.1.162-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J. The effects of octylglucoside on the Semliki forest virus membrane. Evidence for a spike-protein--nucleocapsid interaction. Eur J Biochem. 1980 May;106(2):613–618. doi: 10.1111/j.1432-1033.1980.tb04609.x. [DOI] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefrancois L., Lyles D. S. The interaction of antibody with the major surface glycoprotein of vesicular stomatitis virus. I. Analysis of neutralizing epitopes with monoclonal antibodies. Virology. 1982 Aug;121(1):157–167. [PubMed] [Google Scholar]
- Li Y., Luo L. Z., Snyder R. M., Wagner R. R. Expression of the M gene of vesicular stomatitis virus cloned in various vaccinia virus vectors. J Virol. 1988 Mar;62(3):776–782. doi: 10.1128/jvi.62.3.776-782.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Florkiewicz R. Z., Rose J. K. A single N-linked oligosaccharide at either of the two normal sites is sufficient for transport of vesicular stomatitis virus G protein to the cell surface. Mol Cell Biol. 1985 Nov;5(11):3074–3083. doi: 10.1128/mcb.5.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella D. A., Lenard J. Interactions of wild-type and mutant M protein of vesicular stomatitis virus with viral nucleocapsid and envelope in intact virions. Evidence from [125I]iodonaphthyl azide labeling and specific cross-linking. Biochemistry. 1981 Nov 24;20(24):6872–6877. doi: 10.1021/bi00527a020. [DOI] [PubMed] [Google Scholar]
- Metsikkö K., Simons K. The budding mechanism of spikeless vesicular stomatitis virus particles. EMBO J. 1986 Aug;5(8):1913–1920. doi: 10.1002/j.1460-2075.1986.tb04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti K. G., Webster R. G. Distribution of hemagglutinin and neuraminidase on influenza virions as revealed by immunoelectron microscopy. Virology. 1986 Feb;149(1):36–43. doi: 10.1016/0042-6822(86)90084-x. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. doi: 10.1128/jvi.39.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald W. F., Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Stereo images of vesicular stomatitis virus assembly. J Virol. 1986 Mar;57(3):922–932. doi: 10.1128/jvi.57.3.922-932.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez L. G., Davis G. L., Hunter E. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J Virol. 1987 Oct;61(10):2981–2988. doi: 10.1128/jvi.61.10.2981-2988.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Machamer C. E., Rose J. K. Cytoplasmic domains of cellular and viral integral membrane proteins substitute for the cytoplasmic domain of the vesicular stomatitis virus glycoprotein in transport to the plasma membrane. J Cell Biol. 1986 Jun;102(6):2147–2157. doi: 10.1083/jcb.102.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Adams G. A., Gallione C. J. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2050–2054. doi: 10.1073/pnas.81.7.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W. Delayed appearance of pseudotypes between vesicular stomatitis virus influenza virus during mixed infection of MDCK cells. J Virol. 1981 Dec;40(3):848–860. doi: 10.1128/jvi.40.3.848-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schnitzer T. J., Dickson C., Weiss R. A. Morphological and biochemical characterization of viral particles produced by the tsO45 mutant of vesicular stomatitis virus at restrictive temperature. J Virol. 1979 Jan;29(1):185–195. doi: 10.1128/jvi.29.1.185-195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Simons K., Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Adv Protein Chem. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Vaux D. J., Helenius A., Mellman I. Spike--nucleocapsid interaction in Semliki Forest virus reconstructed using network antibodies. Nature. 1988 Nov 3;336(6194):36–42. doi: 10.1038/336036a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Bennett P. L. Assembly of membrane glycoproteins studied by phenotypic mixing between mutants of vesicular stomatitis virus and retroviruses. Virology. 1980 Jan 30;100(2):252–274. doi: 10.1016/0042-6822(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Whitt M. A., Manning J. S. A phosphorylated 34-kDa protein and a subpopulation of polyhedrin are thiol linked to the carbohydrate layer surrounding a baculovirus occlusion body. Virology. 1988 Mar;163(1):33–42. doi: 10.1016/0042-6822(88)90231-0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Mechanism of formation of pseudotypes between vesicular stomatitis virus and murine leukemia virus. Cell. 1977 Jul;11(3):505–511. doi: 10.1016/0092-8674(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Závada J., Rosenbergová M. Phenotypic mixing of vesicular stomatitis virus with fowl plague virus. Acta Virol. 1972 Mar;16(2):103–114. [PubMed] [Google Scholar]
- Závada J. The pseudotypic paradox. J Gen Virol. 1982 Nov;63(Pt 1):15–24. doi: 10.1099/0022-1317-63-1-15. [DOI] [PubMed] [Google Scholar]