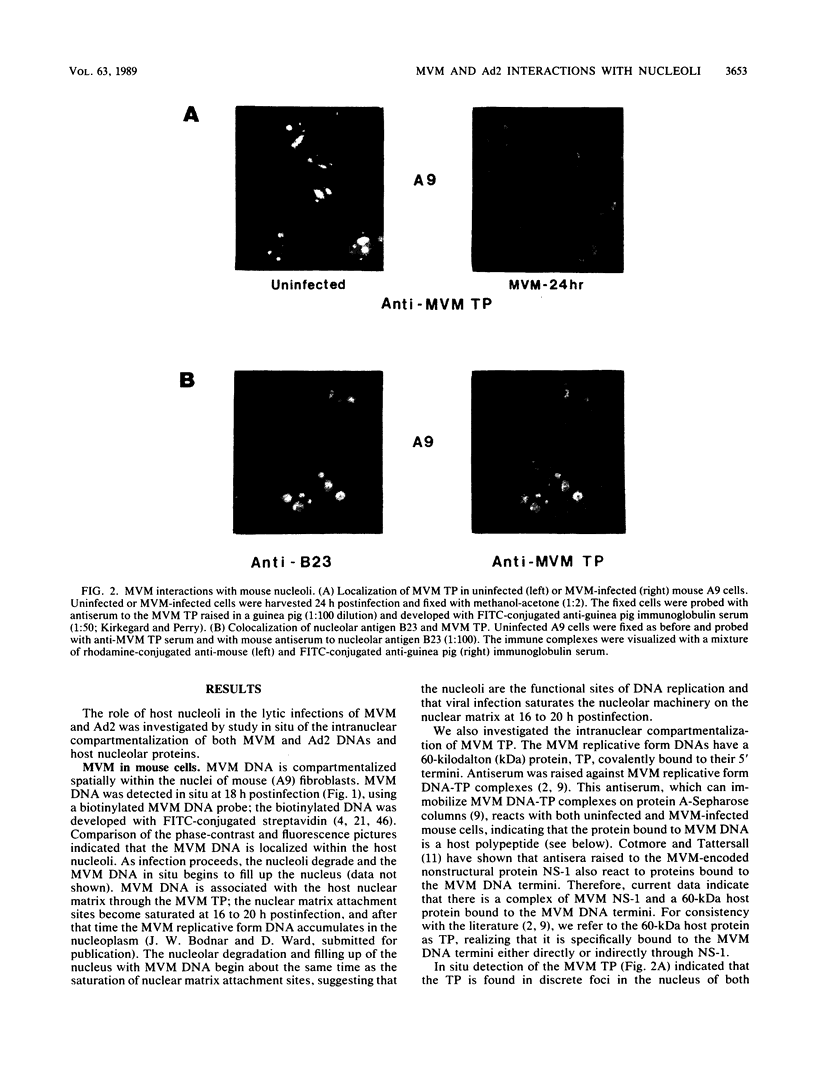
Abstract
Biochemical evidence is presented that both minute virus of mice (MVM) and adenovirus interact with the nucleolus during lytic growth and that MVM can also target specific changes involving nucleolar components in adenovirus-infected cells. These virus-nucleolus interactions were studied by analysis of intranuclear compartmentalization of both viral DNAs and host nucleolar proteins: (i) MVM in mouse cells (its normal host) replicates its DNA in the host nucleoli; (ii) specific nucleolar proteins as well as small nuclear ribonucleoprotein antigens are recompartmentalized to multiple intranuclear foci in adenovirus-infected HeLa cells; and (iii) when adenovirus helps MVM DNA replication in a nonpermissive human cell (HeLa), the MVM DNA is also recompartmentalized for synthesis. The data suggest mechanisms for disruption of nucleolar function common to oncogenic or oncolytic virus lytic growth and cell transformation.
Full text
PDF

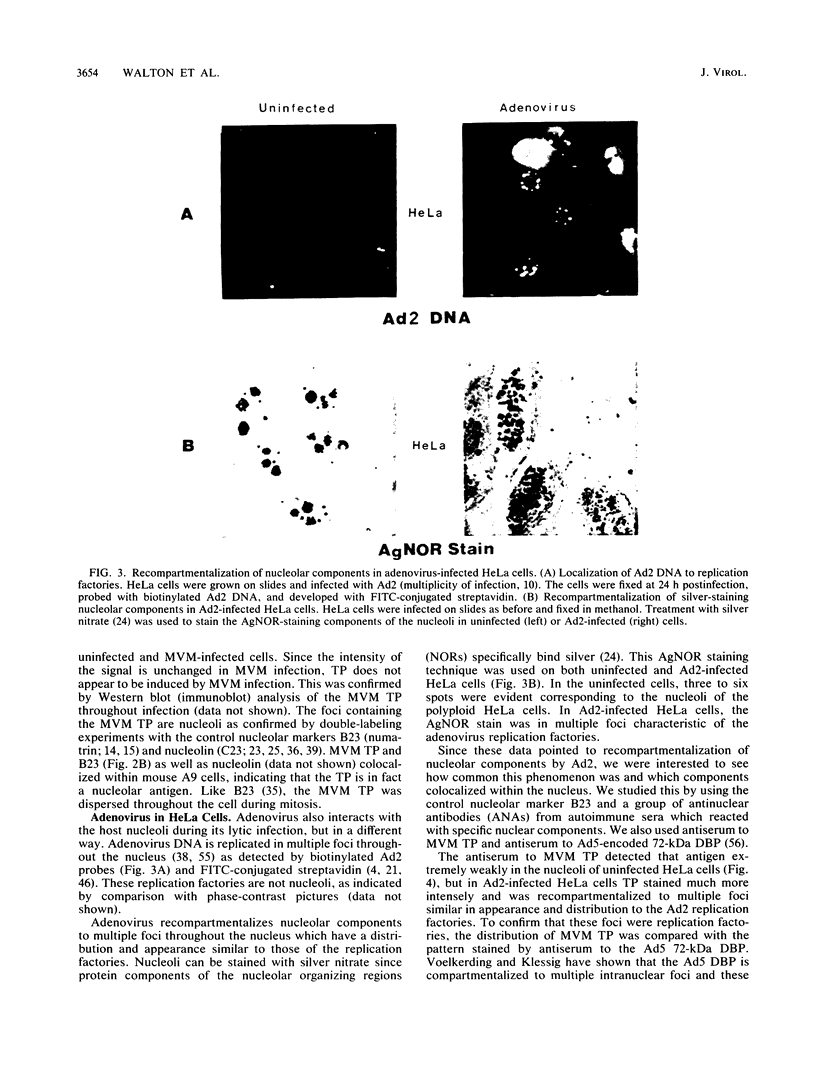
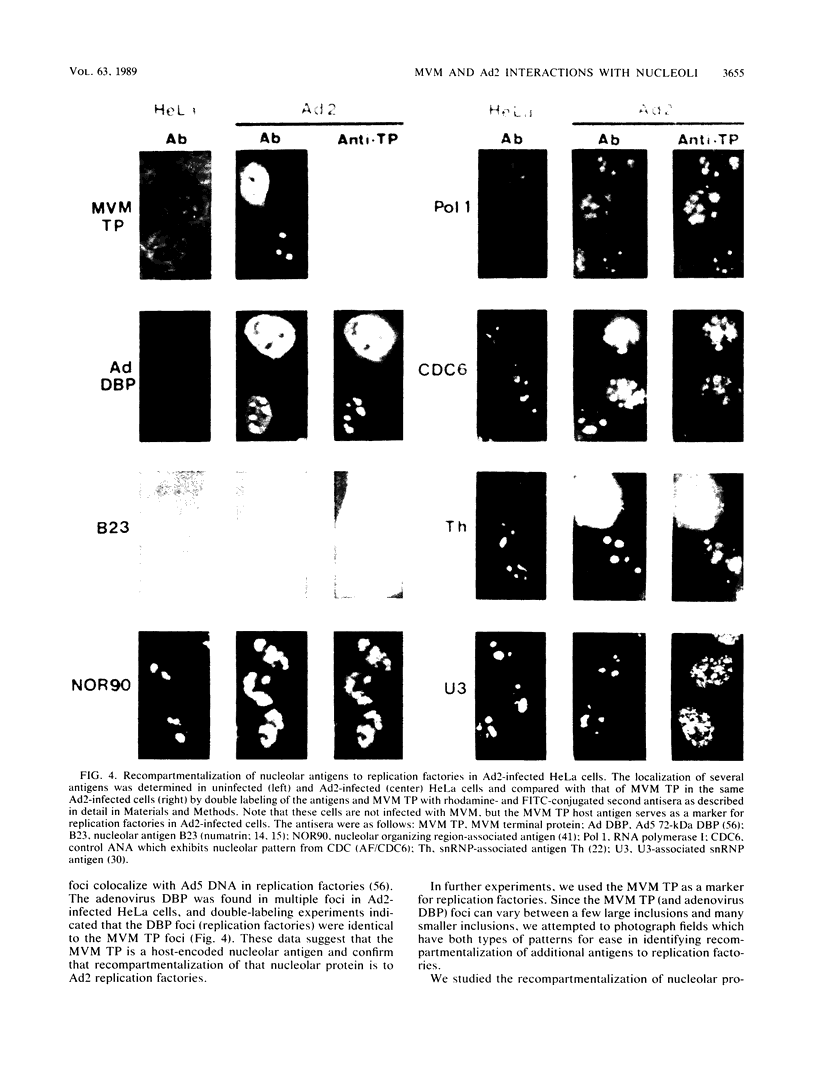
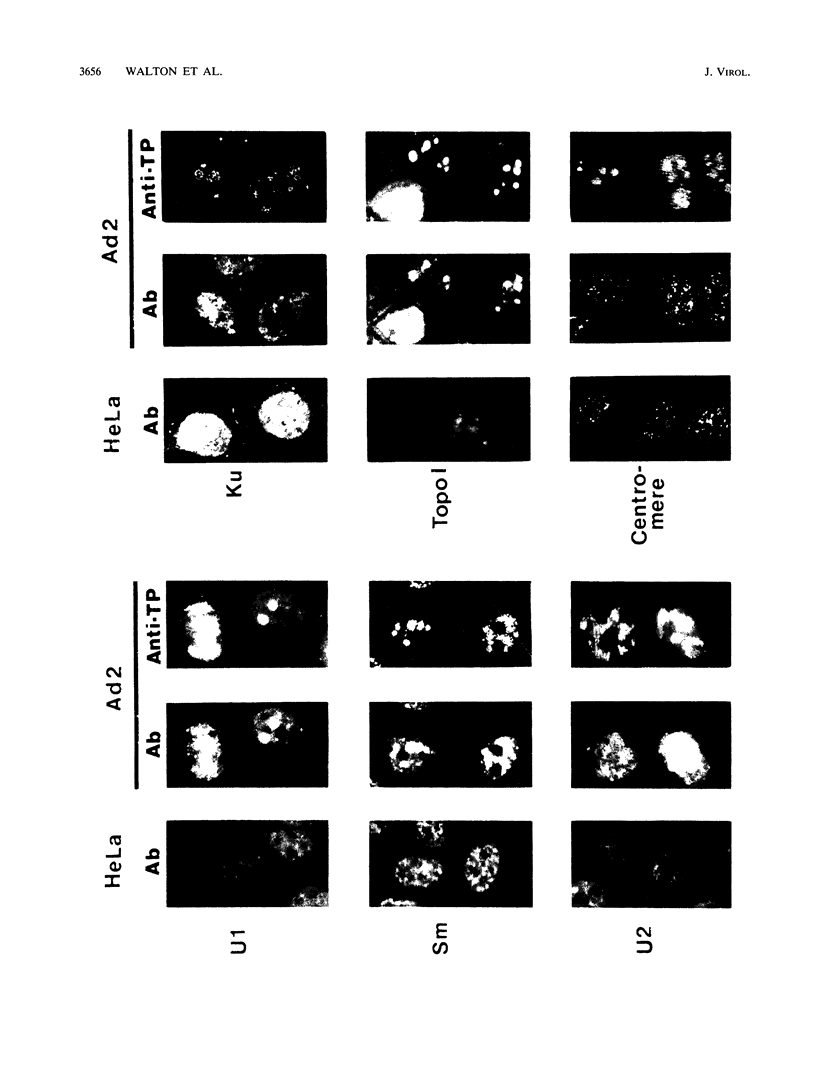




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Thomson M., Chow M. B., Ward D. C. Structure and replication of minute virus of mice DNA. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):751–762. doi: 10.1101/sqb.1983.047.01.086. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Labow M. A. Parvovirus gene regulation. J Gen Virol. 1987 Mar;68(Pt 3):601–614. doi: 10.1099/0022-1317-68-3-601. [DOI] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Castiglia C. L., Flint S. J. Effects of adenovirus infection on rRNA synthesis and maturation in HeLa cells. Mol Cell Biol. 1983 Apr;3(4):662–671. doi: 10.1128/mcb.3.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. K., Frakes R., Tan E. M., Brattain M. G., Smetana K., Busch H. Indirect immunofluorescence studies of proliferating cell nuclear antigen in nucleoli of human tumor and normal tissues. Cancer Res. 1983 Aug;43(8):3770–3777. [PubMed] [Google Scholar]
- Chow K. C., Pearson G. D. Adenovirus infection elevates levels of cellular topoisomerase I. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2247–2251. doi: 10.1073/pnas.82.8.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K. C., Pearson G. D. Site-specific nicking within the adenovirus inverted terminal repetition. Nucleic Acids Res. 1984 Feb 10;12(3):1489–1500. doi: 10.1093/nar/12.3.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow M., Bodnar J. W., Polvino-Bodnar M., Ward D. C. Identification and characterization of a protein covalently bound to DNA of minute virus of mice. J Virol. 1986 Mar;57(3):1094–1104. doi: 10.1128/jvi.57.3.1094-1104.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5' termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988 Mar;62(3):851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Culotta V., Sollner-Webb B. Sites of topoisomerase I action on X. laevis ribosomal chromatin: transcriptionally active rDNA has an approximately 200 bp repeating structure. Cell. 1988 Feb 26;52(4):585–597. doi: 10.1016/0092-8674(88)90471-0. [DOI] [PubMed] [Google Scholar]
- Feldman L. T., Nevins J. R. Localization of the adenovirus E1Aa protein, a positive-acting transcriptional factor, in infected cells infected cells. Mol Cell Biol. 1983 May;3(5):829–838. doi: 10.1128/mcb.3.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein N., Mond J. J. "Numatrin," a nuclear matrix protein associated with induction of proliferation in B lymphocytes. J Biol Chem. 1987 Aug 15;262(23):11389–11397. [PubMed] [Google Scholar]
- Feuerstein N., Spiegel S., Mond J. J. The nuclear matrix protein, numatrin (B23), is associated with growth factor-induced mitogenesis in Swiss 3T3 fibroblasts and with T lymphocyte proliferation stimulated by lectins and anti-T cell antigen receptor antibody. J Cell Biol. 1988 Nov;107(5):1629–1642. doi: 10.1083/jcb.107.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman J. W., Dowell B. L., Ochs R. L., Ross B. E., Busch H. Effect of differentiation on the expression of a nucleolar antigen with a molecular weight of 145,000 in HL-60 cells. Cancer Res. 1987 Jan 15;47(2):586–591. [PubMed] [Google Scholar]
- GRANBOULAN N., TOURNIER P., WICKER R., BERNHARD W. An electron microscope study of the development of SV40 virus. J Cell Biol. 1963 May;17:423–441. doi: 10.1083/jcb.17.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambke C., Deppert W. Late nonstructural 100,000- and 33,000-dalton proteins of adenovirus type 2. I. Subcellular localization during the course of infection. J Virol. 1981 Nov;40(2):585–593. doi: 10.1128/jvi.40.2.585-593.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuskens M., May E. Ultrastructural localization of SV40 viral DNA in cells, during lytic infection, by in situ molecular hybridization. Exp Cell Res. 1974 Jul;87(1):175–185. doi: 10.1016/0014-4827(74)90540-0. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem. 1983 Feb 10;258(3):1379–1382. [PubMed] [Google Scholar]
- Howell W. M., Black D. A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980 Aug 15;36(8):1014–1015. doi: 10.1007/BF01953855. [DOI] [PubMed] [Google Scholar]
- Jordan G. At the heart of the nucleolus. Nature. 1987 Oct 8;329(6139):489–490. doi: 10.1038/329489a0. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Smith J. L. A mutant herpesvirus protein leads to a block in nuclear localization of other viral proteins. Mol Cell Biol. 1986 Jul;6(7):2371–2381. doi: 10.1128/mcb.6.7.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakidis S., Stevely W. S. The effect of herpesvirus infection on ribosomal RNA synthesis and on nucleolar size and number in HeLa cells. Arch Virol. 1982;71(1):79–83. doi: 10.1007/BF01315177. [DOI] [PubMed] [Google Scholar]
- Ledinko N. Nucleolar ribosomal precursor RNA and protein metabolism in human embryo kidney cultures infected with adenovirus 12. Virology. 1972 Jul;49(1):79–89. doi: 10.1016/s0042-6822(72)80008-4. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs R. L., Reddy R., Cook R. G., Yeoman L. C., Tan E. M., Reichlin M., Busch H. Purification and partial characterization of a nucleolar scleroderma antigen (Mr = 34,000; pI, 8.5) rich in NG,NG-dimethylarginine. J Biol Chem. 1985 Nov 15;260(26):14304–14310. [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Repeated genes in eukaryotes. Annu Rev Biochem. 1980;49:727–764. doi: 10.1146/annurev.bi.49.070180.003455. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyne G., Pichard E., Bernhard W. Localization of simian adenovirus 7 (SA 7) transcription and replication in lytic infection. An ultracytochemical and autoradiographical study. J Gen Virol. 1978 Jul;40(1):77–92. doi: 10.1099/0022-1317-40-1-77. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Pfund W. P., Mehta V. B., Trask D. K. Eukaryotic type I topoisomerase is enriched in the nucleolus and catalytically active on ribosomal DNA. EMBO J. 1985 May;4(5):1237–1243. doi: 10.1002/j.1460-2075.1985.tb03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs R., Lischwe M., O'Leary P., Busch H. Localization of nucleolar phosphoproteins B23 and C23 during mitosis. Exp Cell Res. 1983 Jun;146(1):139–149. doi: 10.1016/0014-4827(83)90332-4. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Prestayko A. W., Jones C. F., Busch H. Phosphorylation of proteins of ribosomes and nucleolar preribosomal particles from Novikoff hepatmoa ascites cells. J Mol Biol. 1974 Nov 25;90(1):161–168. doi: 10.1016/0022-2836(74)90264-2. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Phillips D. M., Raskas H. J. Ultrastructural changes in KB cultures infected with adenovirus type 2. Virology. 1972 Apr;48(1):156–169. doi: 10.1016/0042-6822(72)90123-7. [DOI] [PubMed] [Google Scholar]
- Prestayko A. W., Klomp G. R., Schmoll D. J., Busch H. Comparison of proteins of ribosomal subunits and nucleolar preribosomal particles from Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Biochemistry. 1974 Apr 23;13(9):1945–1951. doi: 10.1021/bi00706a026. [DOI] [PubMed] [Google Scholar]
- Richards R., Linser P., Armentrout R. W. Kinetics of assembly of a parvovirus, minute virus of mice, in synchronized rat brain cells. J Virol. 1977 Jun;22(3):778–793. doi: 10.1128/jvi.22.3.778-793.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sanchez J. L., Gelpi C., Juarez C., Hardin J. A. Anti-NOR 90. A new autoantibody in scleroderma that recognizes a 90-kDa component of the nucleolus-organizing region of chromatin. J Immunol. 1987 Oct 15;139(8):2579–2584. [PubMed] [Google Scholar]
- Sasaguri Y., Sanford T., Aguirre P., Padmanabhan R. Immunological analysis of 140-kDa adenovirus-encoded DNA polymerase in adenovirus type 2-infected HeLa cells using antibodies raised against the protein expressed in Escherichia coli. Virology. 1987 Oct;160(2):389–399. doi: 10.1016/0042-6822(87)90010-9. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Ultrastructural studies of H-1 parvovirus replication. VI. simultaneous autoradiographic and immunochemical intranuclear localization of viral DNA synthesis and protein accumulation. J Virol. 1978 Jan;25(1):349–360. doi: 10.1128/jvi.25.1.349-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Toolan H. W. Ultrastructural studies of H-1 parvovirus replication. I. Cytopathology produced in human NB epithelial cells and hamster embryo fibroblasts. Virology. 1975 May;65(1):40–54. doi: 10.1016/0042-6822(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Singer I. I. Ultrastructural studies of H-1 parvovirus replication. III. Intracellular localization of viral antigens with immunocytochrome c. Exp Cell Res. 1976 May;99(2):346–356. doi: 10.1016/0014-4827(76)90592-9. [DOI] [PubMed] [Google Scholar]
- Sirtori C., Bosisio-Bestetti M. Nucleolar changes in KB tumor cells infected with herpes simplex virus. Cancer Res. 1967 Feb;27(2):367–376. [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Soprano K. J., Dev V. G., Croce C. M., Baserga R. Reactivation of silent rRNA genes by simian virus 40 in human-mouse hybrid cells. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3885–3889. doi: 10.1073/pnas.76.8.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Berg C., Hendrick J. P., La Branche-Chabot H., Metspalu A., Rinke J., Yario T. A 5S rRNA/L5 complex is a precursor to ribosome assembly in mammalian cells. J Cell Biol. 1988 Mar;106(3):545–556. doi: 10.1083/jcb.106.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilly W. G. Maintenance of perpetual synchrony in HeLa S3 culture: theoretical and empirical approaches. Methods Cell Biol. 1976;14:273–285. doi: 10.1016/s0091-679x(08)60489-6. [DOI] [PubMed] [Google Scholar]
- Vlak J. M., Rozijn R. H., Spies F. Localization of adenovirus DNA replication in KB cells. Virology. 1975 Jun;65(2):535–545. doi: 10.1016/0042-6822(75)90058-6. [DOI] [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol. 1969 Jul;4(1):36–46. doi: 10.1128/jvi.4.1.36-46.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Spector D., Welch W. Differential distribution of the adenovirus E1A proteins and colocalization of E1A with the 70-kilodalton cellular heat shock protein in infected cells. J Virol. 1988 Nov;62(11):4153–4166. doi: 10.1128/jvi.62.11.4153-4166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung B. Y., Busch H., Chan P. K. Effects of luzopeptins on protein B23 translocation and ribosomal RNA synthesis in HeLa cells. Cancer Res. 1986 Feb;46(2):922–925. [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988 Dec 2;55(5):857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- van der Eb A. J., Bernards R. Transformation and oncogenicity by adenoviruses. Curr Top Microbiol Immunol. 1984;110:23–51. doi: 10.1007/978-3-642-46494-2_2. [DOI] [PubMed] [Google Scholar]