Abstract
Herpes simplex virus (HSV) induces DNA amplification of target genes within the host cell chromosome. To characterize the HSV genes that mediate the amplification effect, combinations of cloned DNA fragments covering the entire HSV genome were transiently transfected into simian virus 40 (SV40)-transformed hamster cells. This led to amplification of the integrated SV40 DNA sequences to a degree comparable to that observed after transfection of intact virion DNA. Transfection of combinations of subclones and of human cytomegalovirus immediate-early promoter-driven expression constructs for individual open reading frames led to the identification of six HSV genes which together were necessary and sufficient for the induction of DNA amplification: UL30 (DNA polymerase), UL29 (major DNA-binding protein), UL5, UL8, UL42, and UL52. All of these genes encode proteins necessary for HSV DNA replication. However, an additional gene coding for an HSV origin-binding protein (UL9) was required for origin-dependent HSV DNA replication but was dispensible for SV40 DNA amplification. Our results show that a subset of HSV replication genes is sufficient for the induction of DNA amplification. This opens the possibility that HSV expresses functions sufficient for DNA amplification but separate from those responsible for lytic viral growth. HSV infection may thereby induce DNA amplification within the host cell genome without killing the host by lytic viral growth. This may lead to persistence of a cell with a new genetic phenotype, which would have implications for the pathogenicity of the virus in vivo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M. Oncogene amplification in tumor cells. Adv Cancer Res. 1986;47:235–281. doi: 10.1016/s0065-230x(08)60201-8. [DOI] [PubMed] [Google Scholar]
- Biswal N., Feldan P., Levy C. C. A DNA topoisomerase activity copurifies with the DNA polymerase induced by herpes simplex virus. Biochim Biophys Acta. 1983 Sep 9;740(4):379–389. doi: 10.1016/0167-4781(83)90086-6. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Carmichael E. P., Kosovsky M. J., Weller S. K. Isolation and characterization of herpes simplex virus type 1 host range mutants defective in viral DNA synthesis. J Virol. 1988 Jan;62(1):91–99. doi: 10.1128/jvi.62.1.91-99.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenet-Monte C., Mohammad F., Celluzzi C. M., Schaffer P. A., Farber F. E. Herpes simplex virus gene products involved in the induction of chromosomal aberrations. Virus Res. 1986 Dec;6(3):245–260. doi: 10.1016/0168-1702(86)90073-0. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute J. J., Mocarski E. S., Lehman I. R. A DNA helicase induced by herpes simplex virus type 1. Nucleic Acids Res. 1988 Jul 25;16(14A):6585–6596. doi: 10.1093/nar/16.14.6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crute J. J., Tsurumi T., Zhu L. A., Weller S. K., Olivo P. D., Challberg M. D., Mocarski E. S., Lehman I. R. Herpes simplex virus 1 helicase-primase: a complex of three herpes-encoded gene products. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2186–2189. doi: 10.1073/pnas.86.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P., Lehman I. R. Interaction of origin binding protein with an origin of replication of herpes simplex virus 1. Proc Natl Acad Sci U S A. 1988 May;85(9):2959–2963. doi: 10.1073/pnas.85.9.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R. D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984 Dec 20;3(13):3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Gallo M. L., Jackwood D. H., Murphy M., Marsden H. S., Parris D. S. Purification of the herpes simplex virus type 1 65-kilodalton DNA-binding protein: properties of the protein and evidence of its association with the virus-encoded DNA polymerase. J Virol. 1988 Aug;62(8):2874–2883. doi: 10.1128/jvi.62.8.2874-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Gerlach J. H., Kartner N., Bell D. R., Ling V. Multidrug resistance. Cancer Surv. 1986;5(1):25–46. [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Hall J. D., Mount D. W., Retondo M. J., Weller S. K., Coen D. M. Sequence and mapping analyses of the herpes simplex virus DNA polymerase gene predict a C-terminal substrate binding domain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7969–7973. doi: 10.1073/pnas.82.23.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L., Sandri-Goldin R. M., Levine M., Glorioso J. C. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol. 1981 Apr;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988 Aug;62(8):2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMPAR B., ELLISON S. A. Chromosomal aberrations induced by an animal virus. Nature. 1961 Oct 14;192:145–147. doi: 10.1038/192145a0. [DOI] [PubMed] [Google Scholar]
- Heilbronn R., Schlehofer J. R., Yalkinoglu A. O., Zur Hausen H. Selective DNA-amplification induced by carcinogens (initiators): evidence for a role of proteases and DNA polymerase alpha. Int J Cancer. 1985 Jul 15;36(1):85–91. doi: 10.1002/ijc.2910360114. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Wietstock S. M., Ruyechan W. T. Identification and characterization of a DNA primase activity present in herpes simplex virus type 1-infected HeLa cells. J Virol. 1988 Mar;62(3):1038–1045. doi: 10.1128/jvi.62.3.1038-1045.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger T., Etkin S., Lavi S. Carcinogen-mediated methotrexate resistance and dihydrofolate reductase amplification in Chinese hamster cells. Mol Cell Biol. 1986 Jun;6(6):1958–1964. doi: 10.1128/mcb.6.6.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberger T., Sahar E., Lavi S. Carcinogen-mediated co-activation of two independent genes in Chinese hamster cells. Carcinogenesis. 1988 Jun;9(6):979–985. doi: 10.1093/carcin/9.6.979. [DOI] [PubMed] [Google Scholar]
- Knopf K. W. Properties of herpes simplex virus DNA polymerase and characterization of its associated exonuclease activity. Eur J Biochem. 1979 Jul;98(1):231–244. doi: 10.1111/j.1432-1033.1979.tb13181.x. [DOI] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. D., Kruper J. A., Frenkel N. Herpes simplex virus virion host shutoff function. J Virol. 1988 Mar;62(3):912–921. doi: 10.1128/jvi.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Tanaka N., Lamb P. W., Gilmer T. M., Barrett J. C. Induction of gene amplification by arsenic. Science. 1988 Jul 1;241(4861):79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Herpes simplex virus and human cytomegalovirus: their role in morphological transformation and genital cancers. J Gen Virol. 1987 Oct;68(Pt 10):2525–2550. doi: 10.1099/0022-1317-68-10-2525. [DOI] [PubMed] [Google Scholar]
- Marchetti M. E., Smith C. A., Schaffer P. A. A temperature-sensitive mutation in a herpes simplex virus type 1 gene required for viral DNA synthesis maps to coordinates 0.609 through 0.614 in UL. J Virol. 1988 Mar;62(3):715–721. doi: 10.1128/jvi.62.3.715-721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Moriuchi T., Koji T., Nakane P. K. Molecular cloning of cDNA coding for rat proliferating cell nuclear antigen (PCNA)/cyclin. EMBO J. 1987 Mar;6(3):637–642. doi: 10.1002/j.1460-2075.1987.tb04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
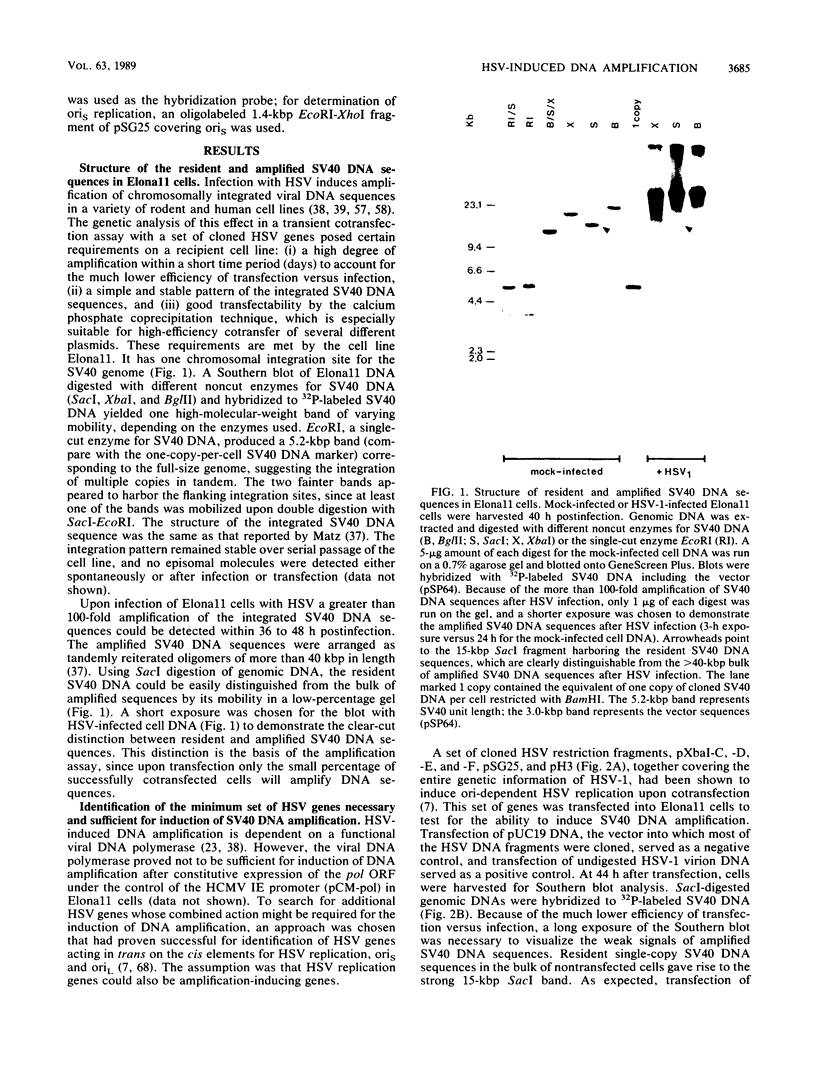
- Matz B. Herpes simplex virus infection generates large tandemly reiterated simian virus 40 DNA molecules in a transformed hamster cell line. J Virol. 1987 May;61(5):1427–1434. doi: 10.1128/jvi.61.5.1427-1434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz B., Schlehofer J. R., Zur Hausen H., Huber B., Fanning E. HSV- and chemical carcinogen-induced amplification of SV40 DNA sequences in transformed cells is cell-line-dependent. Int J Cancer. 1985 Apr 15;35(4):521–525. doi: 10.1002/ijc.2910350416. [DOI] [PubMed] [Google Scholar]
- Matz B., Schlehofer J. R., zur Hausen H. Identification of a gene function of herpes simplex virus type 1 essential for amplification of simian virus 40 DNA sequences in transformed hamster cells. Virology. 1984 Apr 30;134(2):328–337. doi: 10.1016/0042-6822(84)90301-5. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
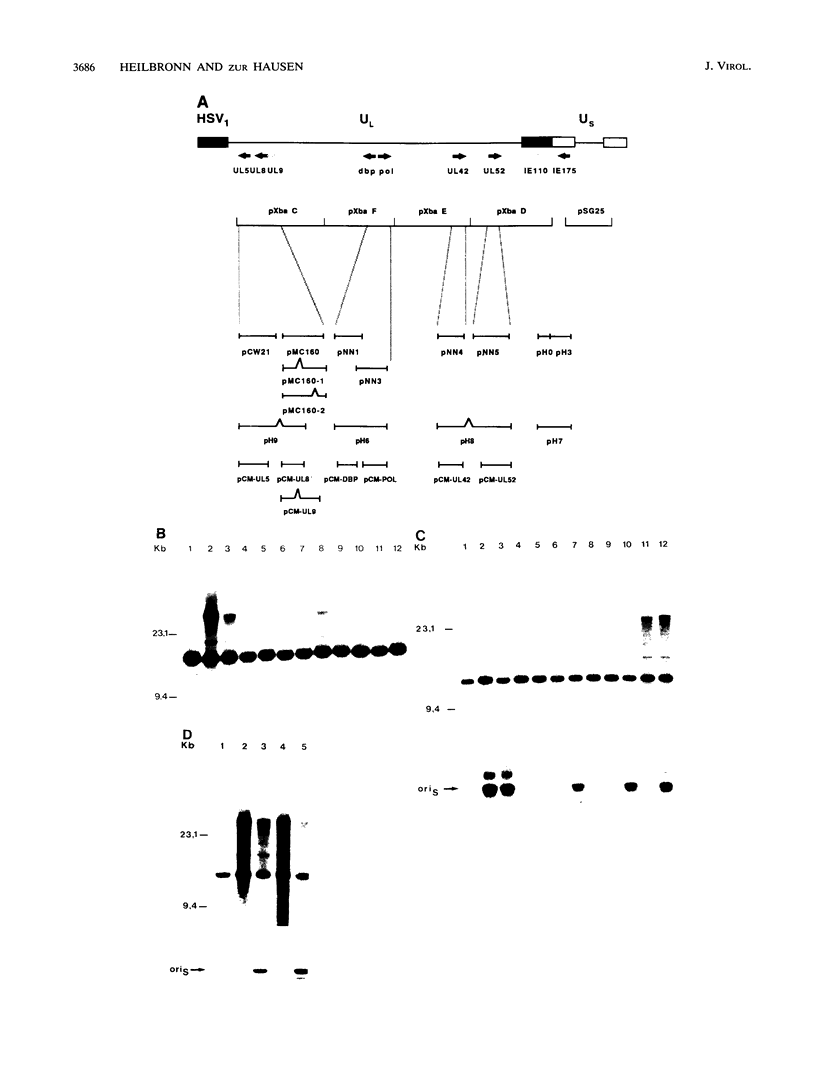
- McGeoch D. J., Dalrymple M. A., Dolan A., McNab D., Perry L. J., Taylor P., Challberg M. D. Structures of herpes simplex virus type 1 genes required for replication of virus DNA. J Virol. 1988 Feb;62(2):444–453. doi: 10.1128/jvi.62.2.444-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Bolles C. S., Parris D. S. Association of type I DNA topoisomerase with herpes simplex virus. J Gen Virol. 1985 Jul;66(Pt 7):1565–1574. doi: 10.1099/0022-1317-66-7-1565. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y., Rapp F. Repair replication of viral and cellular DNA in herpes simplex virus type 2-infected human embryonic and xeroderma pigmentosum cells. Virology. 1981 Apr 30;110(2):466–475. doi: 10.1016/0042-6822(81)90077-5. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. E., Elias P., Funnell B. E., Lehman I. R. Interaction between the DNA polymerase and single-stranded DNA-binding protein (infected cell protein 8) of herpes simplex virus 1. J Biol Chem. 1987 Mar 25;262(9):4260–4266. [PubMed] [Google Scholar]
- O'Hare P., Hayward G. S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985 Mar;53(3):751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo P. D., Nelson N. J., Challberg M. D. Herpes simplex virus DNA replication: the UL9 gene encodes an origin-binding protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5414–5418. doi: 10.1073/pnas.85.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orberg P. K., Schaffer P. A. Expression of herpes simplex virus type 1 major DNA-binding protein, ICP8, in transformed cell lines: complementation of deletion mutants and inhibition of wild-type virus. J Virol. 1987 Apr;61(4):1136–1146. doi: 10.1128/jvi.61.4.1136-1146.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parris D. S., Cross A., Haarr L., Orr A., Frame M. C., Murphy M., McGeoch D. J., Marsden H. S. Identification of the gene encoding the 65-kilodalton DNA-binding protein of herpes simplex virus type 1. J Virol. 1988 Mar;62(3):818–825. doi: 10.1128/jvi.62.3.818-825.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon L., Langelier Y., Royal A. Herpes simplex virus type 2 mutagenesis: characterization of mutants induced at the hprt locus of nonpermissive XC cells. Mol Cell Biol. 1986 Aug;6(8):2977–2983. doi: 10.1128/mcb.6.8.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon L., Royal A., Langelier Y. Increased mutation frequency after herpes simplex virus type 2 infection in non-permissive XC cells. J Gen Virol. 1985 Feb;66(Pt 2):259–265. doi: 10.1099/0022-1317-66-2-259. [DOI] [PubMed] [Google Scholar]
- Prelich G., Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988 Apr 8;53(1):117–126. doi: 10.1016/0092-8674(88)90493-x. [DOI] [PubMed] [Google Scholar]
- Quinlan M. P., Knipe D. M. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985 May;5(5):957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J. P., McGeoch D. J. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 1985 Nov 25;13(22):8143–8163. doi: 10.1093/nar/13.22.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T. The major herpes simplex virus DNA-binding protein holds single-stranded DNA in an extended configuration. J Virol. 1983 May;46(2):661–666. doi: 10.1128/jvi.46.2.661-666.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Methotrexate resistance and gene amplification. Mechanisms and implications. Cancer. 1986 May 15;57(10):1912–1917. doi: 10.1002/1097-0142(19860515)57:10<1912::aid-cncr2820571004>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Gissmann L., Matz B., zur Hausen H. Herpes simplex virus-induced amplification of SV40 sequences in transformed Chinese hamster embryo cells. Int J Cancer. 1983 Jul 15;32(1):99–103. doi: 10.1002/ijc.2910320116. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Hausen J. Z. Induction of mutations within the host cell genome by partially inactivated herpes simplex virus type 1. Virology. 1982 Oct 30;122(2):471–475. doi: 10.1016/0042-6822(82)90247-1. [DOI] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Tlsty T. D., Brown P. C., Schimke R. T. UV radiation facilitates methotrexate resistance and amplification of the dihydrofolate reductase gene in cultured 3T6 mouse cells. Mol Cell Biol. 1984 Jun;4(6):1050–1056. doi: 10.1128/mcb.4.6.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waubke R., Zur Hausen H., Henle W. Chromosomal and autoradiographic studies of cells infected with herpes simplex virus. J Virol. 1968 Oct;2(10):1047–1054. doi: 10.1128/jvi.2.10.1047-1054.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., de Villiers J., Schaffner W. An SV40 "enhancer trap" incorporates exogenous enhancers or generates enhancers from its own sequences. Cell. 1984 Apr;36(4):983–992. doi: 10.1016/0092-8674(84)90048-5. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Challberg M. D., Nelson N. J., Levine M., Glorioso J. C. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell. 1988 Jul 29;54(3):369–381. doi: 10.1016/0092-8674(88)90200-0. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Spadaro A., Schaffer J. E., Murray A. W., Maxam A. M., Schaffer P. A. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985 May;5(5):930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Weller S. K. UL5, a protein required for HSV DNA synthesis: genetic analysis, overexpression in Escherichia coli, and generation of polyclonal antibodies. Virology. 1988 Oct;166(2):366–378. doi: 10.1016/0042-6822(88)90507-7. [DOI] [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988 Dec 2;55(5):857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. The role of viruses in human tumors. Adv Cancer Res. 1980;33:77–107. doi: 10.1016/s0065-230x(08)60669-7. [DOI] [PubMed] [Google Scholar]