Abstract
Addressing health disparities has been a national challenge for decades. The National Institutes of Health–sponsored Centers for Population Health and Health Disparities are the first federal initiative to support transdisciplinary multilevel research on the determinants of health disparities. Their novel research approach combines population, clinical, and basic science to elucidate the complex determinants of health disparities. The centers are partnering with community-based, public, and quasi-public organizations to disseminate scientific findings and guide clinical practice in communities. In turn, communities and public health agents are shaping the research. The relationships forged through these complex collaborations increase the likelihood that the centers’ scientific findings will be relevant to communities and contribute to reductions in health disparities.
ADDRESSING HEALTH DISPARITIES AS A PUBLIC HEALTH GOAL
Although the Report of the Secretary’s Task Force on Black and Minority Health was published in 1985 by the US Department of Health and Human Services,1 racial and ethnic disparities in health care were seriously addressed only with the 1999 publication of Healthy People 2010.2,3 At that time, the Centers for Disease Control and Prevention introduced the Racial and Ethnic Approaches to Community Health program, which features community-based participatory research as a tool to achieve social justice in health care.2,3
When the National Center on Minority Health and Health Disparities was established as part of the Minority Health and Health Disparities Research and Education Act of 2000, all National Institutes of Health (NIH) institutes and centers were required to develop strategic plans for modifying and eliminating health disparities. This initiative incorporated into the federal public health discourse the idea that disparities in health outcomes are not inevitable. Federally funded researchers were challenged to adopt research models combining social, behavioral, clinical, and basic science.4 Reports published by the Institute of Medicine, the National Academy of Sciences also described new multilevel, transdisciplinary research paradigms that integrated theories from the social and behavioral sciences with new research on genetics and molecular biology, thus expanding the scientific understanding of determinants of health. Together, all these developments created a strong stimulus for a new approach to research by the federal government.2,3,5–14
The National Institute of Environmental Health Sciences and the National Cancer Institute, National Institute on Aging, and Office of Behavioral and Social Sciences initiated a broad NIH effort to study the determinants of population health disparities. In April 2002, the National Institute of Environmental Health Sciences invited applications to establish Centers for Population Health and Health Disparities (CPHHDs).15 Eight CPHHDs were launched in September 2003. Their primary goals were to create new paradigms to explore the determinants of health disparities and to develop and conduct multilevel, trans-disciplinary research combining population, social and behavioral, clinical, and biological theory and methods. At the same time, the Agency for Healthcare Research and Quality initiated several programs that addressed disparities in health services, so this focus was excluded from the CPHHD initiative.16
Reflecting the design of the Racial and Ethnic Approaches to Community Health program, each CPHHD was required to include at least 1 community-based participatory research project to ensure that resulting new interventions would have external validity and relevance to public health concerns and policy. Participating community-based organizations included groups from inside and external to the universities and at many levels from community to government (Table 1 ▶). All had either successfully organized to address community priorities or shaped and defined policies influencing disparate health outcomes. Participation by community-based organizations in each center’s research and outreach programs fosters local ownership and institutionalization of proven strategies. Partnerships forged through local public health programs, advisory boards, and the direct participation of community members in the research process have expanded the research and service delivery infrastructure at the state and local level.
TABLE 1—
Partnerships Developed by the Centers for Population Health and Health Disparities: 2003–2008
| Centers and Their Partners at Other Universities | Partners in Other University Departments | Government and Quasi-Government Partners | Community-Based Organization Partners |
| Ohio State University and University of Michigan | |||
| University of Kentucky | Ohio State Agricultural Extension Service | Ohio Department of Breast Health and Cervical Cancer | Ohio Division, American Cancer Society |
| Ohio University | College of Public Health College of Medicine Economics Department Psychology Department Comprehensive Cancer Center |
National Cancer Institute Cancer Information Service Centers for Disease Control and Prevention |
Appalachia Community Cancer Network and four community coalitions |
| RAND Corp | |||
| University of Michigan | Los Angeles Department of Park and Recreation | Multicultural Area Health Education Center | |
| University of California, Berkeley | District of Columbia City Council | District of Columbia Primary Care Association | |
| University of California, Los Angeles | |||
| Rutgers University | |||
| Tufts University and Northeastern University | Jean Mayer, USDA, Human Nutrition Research Center on Aging at Tufts University Tufts Friedman School of Nutrition Science and Policy Tufts School of Medicine Northeastern University Department of Sociology Northeastern Center for Urban Health Research |
USDA Agricultural Research Service Massachusetts Department of Public Health |
La Alianza Hispana Tufts–New England Medical Center |
| University of Chicago | |||
| University of Ibadan, Nigeria | School of Social Service Administration | John H. Stroger Jr Hospital of Cook County, IL | Faith Based Wellness Network |
| Ohio State University, RAND Corp, Tufts/Northeastern University, University of Illinois at Chicago, University of Texas Medical Branch, Wayne State University, University of Pennsylvania | Center of Excellence in Health Promotion Economics (CDC) Biological Sciences Division Social Sciences Division Institute for Mind and Biology Robert Wood Johnson National Program Office for Health Disparities Solutions |
Mt Sinai Hospital of Chicago Methodist Hospitals of Gary, IN |
|
| University of Illinois at Chicago | |||
| University of Chicago, Wayne State University, University of Pennsylvania, Rand Corp, Tufts/Northeastern | Institute for Research on Race and Public Policy Midwest Latino Health Research Training and Policy Center International Center for Health Leadership Development University of Illinois at Chicago Cancer Center School of Public Health College of Nursing Department of Sociology Vice Chancellor for Research Institute for Health Research and Policy Survey Research Laboratory |
Illinois Department of Public Health–Illinois State Cancer Registry Chicago Department of Public Health John H. Stroger Jr Hospital of Cook County Illinois State Cancer Plan Institute for Health Care Quality (Medicare) |
Healthcare Consortium of Illinois (Greater Roseland Health District and Healthy South Chicago) Cook County Breast Health Consortium Illinois Division-American Cancer Society |
| University of Pennsylvania | |||
| Cheikh Anta Diop University, Dakar, Senegal | Robert Wood Johnson Health and Society Scholars Program Leonard Davis Institute for Health Economics Abramson Cancer Center Institute on Aging Wharton School of Business Annenberg School of Communication School of Social Work Law School |
US Veterans Administration Hospitals | National Physician and Family Referral Project Philadelphia Chapter, National Black Leadership Initiative on Cancer |
| University of Texas Medical Branch | |||
| University of Maryland Population Center | School of Nursing | Area Health Education Center | Liberty County Cancer Awareness Network |
| University of Texas, Austin Population Research Center | Department of Preventive Medicine and Community Health | Social Security Administration | Parent-Teacher Association of Los Angeles Morgan School |
| Baylor College of Medicine Department of Medicine, Health Services Research | Infectious Disease Obstetrics and Gynecology Division |
Galveston County Health District National Center for Health Statistics–Mortality Division |
Galveston County Cancer Coalition Jesse Tree |
| Wayne State University | |||
| University of Michigan Inter-University Consortium for Political and Social Research | African American Initiative for Male Health Improvement (Henry Ford Hospital) | Detroit Department of Public Health Detroit Medical Center |
Movement for Life Initiative |
| Case Western Reserve University | Minority Center for Urban African American Aging Research College of Nursing College of Liberal Arts Center for Urban Studies Karmanos Cancer Institute Wayne State University Community Relations |
American Heart Association/Detroit Healthy Black Elders Project Health Living Metropolitan Christian Council |
|
Note. USDA = US Department of Agriculture; CDC = Centers for Disease Control and Prevention.
A POPULATION HEALTH APPROACH TO HEALTH DISPARITIES
To elucidate health disparities, research must focus on the determinants of disparate health outcomes across populations.17–22 The recent Institute of Medicine review of the national plan for addressing disparities differentiated between disparities as inequities and differences in population health.4,23 Inequitable health outcomes result from inequities in the distribution of or access to resources that promote good health outcomes; differences refer to outcomes that are the result of biological risk or other factors that are not a matter of policy or discrimination in access. A difference may become a disparity when some subgroups and not others are given access to resources to manage their differential risk from biology or other factors and the groups without access have poorer outcomes. Thus, differences and disparities may have different determinants requiring different forms of intervention.4,23
Population-level determinants of health outcomes are distinct from the determinants of individual health outcomes. Determinants of population health are expressed as rates, averages, and distributions of population characteristics, such as aggregate poverty, education levels, gender and racial/ethnic distributions, and patterns of segregation.17–22 Individuals have risk factors, such as household or individual income, educational attainment, behavior, heredity, and genes. It has been shown that population characteristics affect health outcomes independently of the characteristics of individuals. For example, in studies by Marmot et al., the social status gradient was a population-level determinant of the distribution of health outcomes in a population of British civil servants. In several analyses, the researchers showed that social status defined by the gradient had an effect on individual risk of heart disease onset that was independent of an individual’s biological risk factors.20,21
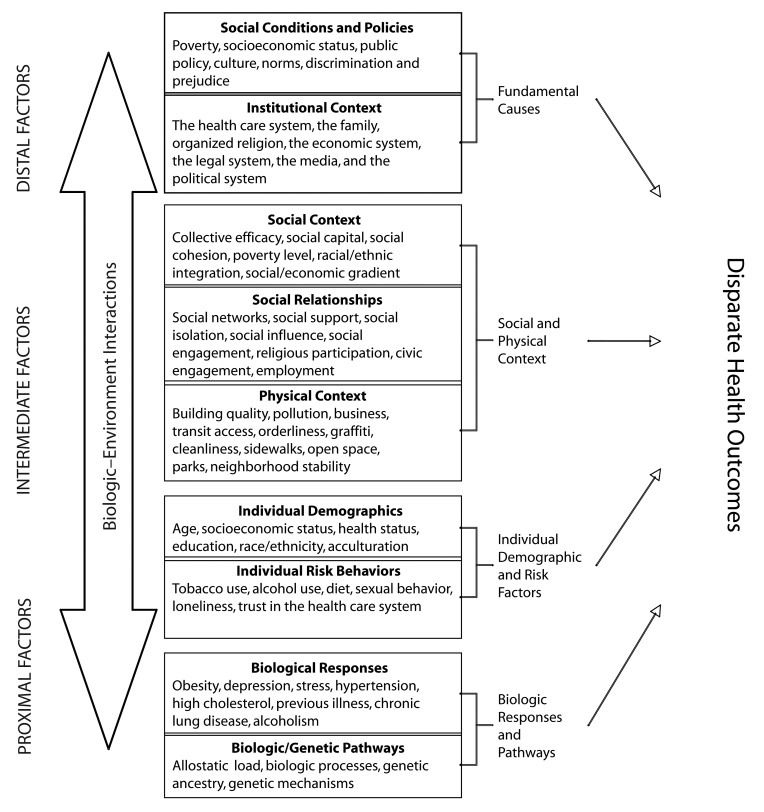
An underlying question addressed by CPHHD centers was phrased by Taylor et al. as, “How does the unhealthy environment get under the skin?”24(p411) In other words, how does population risk relate to individual risk? We drew on the works of Berkman et al.,25 Glass and McAtee,26 and Taylor et al.24 to design our model for approaching this question from a multilevel perspective (Figure 1 ▶).27 Three primary types of determinants are shown on the left side of the figure. Distal determinants include the population social conditions, policies that affect social conditions, and the policymaking bodies that influence or determine them. They are considered fundamental causes because their influence is solely reflected at the population level in the variation in rates of disease or poor health, such as the epidemic of HIV in Africa or rates of obesity in the United States. Their roots are embedded in policy, shared social norms about health and social practices, socioeconomic disadvantage, and policies that affect public availability of health services, including who receives them and the level and quality of service. They are the determinants of inequities rather than differences.28
FIGURE 1—
Model for analysis of population health and health disparities.
Intermediate determinants, the second level of our model, include the immediate social and physical contexts and social relationships in which the distal effects are experienced, such as the community or neighborhood.29,30 The social context includes neighborhood or community poverty level, extent of residential segregation, median income and education, and opportunities for social interaction to redress the effect of the distal factors.31–36 Social relationships include social networks, social engagement, and social influence and are forms of social capital that suppress the negative effects of impoverished social environments. These negative effects can, in the absence of such networks, be increased by social isolation.37–44 The physical environment includes availability and accessibility of local health care resources to the public; availability of transportation, quality air and water, and healthy food; presence of crime; degree of neighborhood disorder; and quality of the built environment.34,35,45–47 We hypothesize that these intermediate determinants are the links through which the environment affects individual demographic factors and risks as well as biological responses and pathways, which compose the proximal determinants. Proximal determinants refer to individuals. Demographic factors characterize both contexts and individuals and in the model can have independent effects. Risk factors and biological responses and pathways refer only to individuals.
Individual determinants include socioeconomic status (SES), race/ethnicity, gender, and level of acculturation. These determinants affect individuals’ capacity to respond to environmental challenges and include where they live, their capacity to address health care needs, the degree to which they have social support, and their level of social integration or isolation. Behaviors such as dietary and sexual practices, exercise, and tobacco use are also individual-level determinants, as are cultural beliefs, which mediate behavior and the capacity to respond to health needs.48,49
In addition to risk behaviors and individual demographics, proximal determinants include biological markers or processes that result from behavior or intermediate determinants and may include risk from heredity or spontaneous mutation or environmental stress. Markers include elevated cholesterol or other indicators of prolonged or intense stress, such as body mass index, high blood pressure, abnormal cells in the cervix, or a lump in the breast.50–57
CPHHD research employs a well-recognized model of how environmental context affects individual health outcomes. The model assumes that the capacity to respond to differences and disparities in the distribution of health outcomes—and the ultimate effectiveness of that response—requires interventions capable of addressing both contextual and individual factors.
APPLYING TRANSDISCIPLINARY STRATEGIES TO UNDERSTANDING HEALTH DISPARITIES
CPHHD investigators at the Center for Interdisciplinary Health Disparities Research (CIHDR) at the University of Chicago have studied both nonhuman animal and human models to elucidate the interactions between the social environment and biology. They established that socially isolated rats developed an acquired vigilant state and died with mammary tumors at younger ages than did their group-housed peers,58 a process that was confirmed in a second project that used SV-40 T-antigen mice. The preliminary conclusion is that the stress of social isolation in nonhuman animals promotes tumor development by activating stress hormone receptors and ultimately preventing the death of malignant cells.59 The nonhuman animal studies enabled CIHDR investigators to isolate a multifaceted suite, made up of depression, loneliness, and vigilance, that is linked to the physiological stress system that is secondary to social isolation. These results suggest directions in which the research can move to better understand these pathways and explore strategies for tumor prevention and therapy.28–30
From Animal to Human Models
The CPHHDs are using our model and findings from the CIHDR to assess the pathways by which the environment may affect health and health status. Although there is an extensive literature on how the environment and social isolation relate to poor health outcomes, this research has not focused on pathways of differences or disparities in health outcomes.41,44,50,51,54,60,61
The results of work with animal models informed the CIHDR investigators’ model of downward causation from social environmental conditions to psychosocial factors, biological responses, and breast tumor development in newly diagnosed African American women living in Chicago. The investigators used in-home interviews, investigations of the built environment, and publicly available community health data geocoded to the study participants’ addresses to uncover a pathway with significant associations between community-level factors and individual characteristics as minute as the genetic information in the nucleus of a cell.
At the community level, dilapidated housing, crime, and general social disorganization lead to isolation, physical assault, and depression. This environment alters the stress hormone response (measured as nighttime rise of cortisol levels, including awakening response). Investigators have identified glucocorticoid receptors in tumors, as well as serum- and glucocorticoid-inducible kinase 1, which is involved in cell survival. Expression of this enzyme occurs when stress hormone travels across the cell membrane and binds with the glucocorticoid receptor complex, creating an environment conducive to cancer growth. This is a possible route by which external, community-level conditions may play a role in the occurrence of cancer.24
Environmental Stressors and Biological Response
These preliminary results have stimulated investigators at other CPHHDs to examine the relationship between physiological stress and health outcomes. For example, investigators at Ohio State University are conducting a case–control study to examine how social, behavioral, and biological factors contribute to the elevated risk of developing cervical abnormalities among Appalachian women. One research aim is to use surrogate markers of chronic Epstein-Barr virus infection to identify molecular markers associated with environmental stressors leading to cervical abnormalities. Preliminary data indicate that Epstein-Barr antigen titers are significantly higher among women with cervical abnormalities. In a stratified analysis adjusted for age, region, and presence of human papilloma virus, the investigators found a significant inverse relationship between SES and levels of Epstein-Barr antigen titers. Borderline statistically significant differences were observed between women at high risk for developing cervical cancer and those with human papilloma virus infection.
At the University of Texas Medical Branch in Galveston, investigators are linking social, geographical, behavioral, and biological data that will contribute to better understanding of the interplay between stress and health among Hispanics. The Hispanic population exhibits the paradox of high morbidity and disability but unexpectedly low mortality, even though Hispanics are clearly disadvantaged in income, education, and access to health care. The investigators analyzed Texas and California vital statistics registries from 1999 to 2001 linked to the 2000 Census and contributed to mounting evidence that mortality rates are substantially lower among Hispanic immigrants, particularly at older ages, than among non-Hispanic Whites but are similar among US-born Hispanics and non-Hispanic Whites.62,63
To better understand the social and biological stress processes that contribute to these differences, the Texas researchers studied a cohort of Hispanics, non-Hispanic African Americans, and non-Hispanic Whites living close to a large petrochemical complex. Preliminary analyses suggested that lower-SES groups, non-Hispanic African Americans, and foreign-born Hispanics exhibited greater concern about refinery danger and greater change in mental health scores after a refinery explosion. The respondents had similar patterns of disparities in biological stress markers. For example, in a case–control study that followed the protocol used by the Ohio State University center, the Texas CPHHD researchers also found evidence that reactivation of latent Epstein-Barr viruses (a marker for immune dysregulation) was associated with lower SES and minority ethnic status. Elevated levels of interleukin-6 and interleukin-10, which signal inflammatory stress response, were associated with distance from a petrochemical complex in multivariate models accounting for age, gender, ethnicity, and education. These early findings suggest that SES and ethnic disparities in stress and health are consistent across psychosocial and biological analyses.64,65
Contextual Stressors and Health Outcomes
Cumulative biological stress, or allostatic load, describes the human body’s physiological response to its environment over time.61 This stress is manifested in dysregulation in multiple physiological systems, including metabolic, cardiac, and inflammatory. Investigators at the RAND Corporation are using National Health and Nutrition Examination Survey III data merged with census tract data to study associations between environmental and biological characteristics. They are analyzing neighborhood or census tract characteristics to elucidate the effects of social, economic, and racial segregation on allostatic load. Preliminary results indicate that low neighborhood SES is associated with increased individual-level allostatic load, even after individual characteristics are controlled.
Neighborhood influences tend to operate differently for men and women. Women living in neighborhoods with low household incomes, a high percentage of male unemployment, and a high percentage of households on public assistance were found to experience increased allostatic load. Men living in neighborhoods with a high percentage of African American households had high allostatic loads. High allostatic loads also were found in neighborhoods with a high proportion of adults younger than 25 years and with less than a high school education. Further, although these effects differed for men and women, they appeared to be similar among Whites, African Americans, and Mexican Americans, suggesting that poverty and segregation experienced in the immediate environment (neighborhood) affect allostatic load independently of race or ethnicity.
Investigators at the University of Illinois at Chicago examined the effects of upward neighborhood SES change on the probability of distant metastasis at diagnosis of breast cancer.66 The researchers analyzed data from 1137 Cook County census tracts from the 1990 and 2000 censuses and data from the Illinois State Cancer Registry for 21516 female breast cancer cases diagnosed in these census tracts from 1994 to 2000. They constructed a multilevel model of 1990 baseline SES of neighborhoods and degree of neighborhood change from 1990 to 2000 (compositional characteristics) and patient’s age and race or Hispanic status (individual characteristics) to predict distant metastasis (vs local and regional stage) at cancer diagnosis. Residence in a census tract experiencing SES improvement was associated with increased odds of distant metastasis at diagnosis, as were being African American and residence in a census tract with lower baseline SES in 1990. Paradoxically, both measures of initial neighborhood disadvantage and upward neighborhood SES change were independently associated with greater odds of the metastasis outcome, suggesting that even in socioeconomically distressed areas, disruption of the social and physical environment may worsen health outcomes, at least in the variable measured.
The investigators conducted a second analysis, with the same data, of breast cancer stage at diagnosis. They used an ordered logistic regression model rather than an arbitrary dichotomization (e.g., late stage vs others) and a random-effects model that accounted for the nesting of cases within census tracts. This analysis disentangled the effects of race/ethnicity and poverty and found that the effects of race/ethnicity diminished with age and completely disappeared after age 60 years, although poverty remained a predictor of late-stage diagnosis. Investigators at 5 other CPHHDs are collaborating with the Chicago researchers to compare the results of both studies with similar analyses from their own locations.
Biological Stress Responses
Investigators at Tufts and Northeastern universities are examining the effect of life experiences and psychosocial stress on allostatic load as a marker of biological risk among Puerto Ricans 65 years and older in the greater Boston area. This population was shown to suffer from an excess burden of chronic conditions, including type 2 diabetes, depression, and physical disability, compared with non-Hispanic Whites of similar age and living in similar neighborhoods.53,57,67 In a study with a prospective cohort design, the researchers are exploring how the relationship of psychosocial stress and its effect on allostatic load affects previously identified health disparities in depression, cognitive impairment, and functional limitation in the same population. They hypothesize that the association between life stress, physiological response, and chronic conditions is modified by biological effects. The investigators are measuring markers of inflammation, hypothesized to be key mediators of the associations of dietary intake and nutritional status with environmental social support. They are also investigating how these associations are modified by genetic variability.
Researchers at Wayne State University’s Center for Urban and African American Health (Detroit, MI) are investigating the effects of vitamin D on obesity and breast cancer recurrence among African American women. They are studying obesity-linked influences on oxidative stress and how such stress affects nitric oxide metabolism, leading to salt-induced rises in blood pressure that may contribute to heightened risk for cancer recurrence in breast cancer survivors.
The investigators in Detroit are also exploring how vitamin D and elevated parathyroid hormone contribute to weight gain. Preliminary analyses found that participants with low vitamin D levels, with or without elevated parathyroid hormone levels, had more body fat than did those with normal vitamin D and parathyroid hormone levels. Vitamin D has antiproliferative and anti-inflammatory properties and may affect body composition, cellular proliferation, and vascular function. The investigators hypothesize that vitamin D may have an important role in causing or mediating high levels of oxidative stress in ductal fluid in breast cancer survivors. Alone or with parathyroid hormone, vitamin D may be involved in mediating the known link between weight gain and breast cancer recurrence.
Gene–Environment Interactions
Variations in neighborhood SES were included in a study of the interaction of genes and environment in prostate cancer at the CPHHD at the University of Pennsylvania. Investigators there have begun to expand the definition of environment in genotype and environment studies to include both individual-and neighborhood-level risk factors. They evaluated the joint relationship of income and education level in residential census tracts with genetic effects on prostate cancer in 1002 White men with prostate cancer and a control group of 387 men.68 Participants’ addresses were geocoded to 2000 Census tracts. The researchers used the tract data to group participants by per capita income, poverty, and educational attainment. The interaction of these neighborhood factors with genotypes of the androgen receptor CAG repeats and SRD5A2-V89L or SRD5A2-A49T variants were evaluated to determine their joint effect on prostate cancer risk and severity. Statistically significant interactions between SRD5A2-V89L genotypes and census tract per capita income were associated with later-stage prostate tumors.
In addition, a significant genotype–neighborhood interaction was observed for androgen receptor CAG genotypes among men living in areas with a high percentage of high school graduates compared with men residing in tracts with a low percentage of high school graduates. These findings suggest that genetic effects on prostate tumor severity are affected by neighborhood characteristics, including poverty and educational attainment. Further, the results indicate that research on genotypes should include neighborhood context.
CONCLUSIONS
Collaborations among 8 CPHHDs across the United States reflect a new approach to elucidating the determinants of health disparities. This research explores the question of “how environment gets under the skin.”24(p411) A preliminary finding is that the neighborhood context has a significant effect on individual risk that is independent of individual characteristics.64,66 If confirmed, this information could inform policies on the availability of screening and treatment facilities.
The NIH Roadmap for Medical Research is designed to encourage studies that fill the gaps in biomedical research and to promote collaboration across NIH agencies. The roadmap aims to significantly affect the progress of biomedical research, disseminate new scientific findings, and transform them into tangible benefits for American populations.69 The principal elements include (1) interdisciplinary teams that cover the complete spectrum of research (basic, clinical, and social and behavioral science), (2) expanded efforts to include minority and medically underserved communities, (3) new partnerships with private and public health care organizations, and (4) enriched educational environments for training the next generation of researchers in the complexities of translating research from bench to bedside and from behavior to policy. All of these elements are key components of the CPHHDs, positioning the centers’ work at the cutting edge of research sponsored by NIH.
CPHHD research to date is almost exclusively cross-sectional, but elucidating health disparities ultimately will require longitudinal studies that examine the entire life span. University of Chicago investigators are employing a lifespan approach in rat models; a next step is to move from preliminary nonhuman animal models to the population level. The animal models are transdisciplinary and multilevel and have the potential to elucidate poorly understood population-level determinants of disparities.
Ultimately, public health policies and resources dedicated to service delivery must be the focus of attempts to eliminate disparities. CPHHDs have adopted the recommendations of the NIH Roadmap for Medical Research, which calls for combining clinical, basic, and social science research to examine the relationships between policy, the social and physical environment, and disparities in health outcomes. The preliminary results described here illustrate the need for multilevel interventions that address context as a key pathway to improving health outcomes. These outcomes are most likely to be seen in clinical and public health practice.
Thus, the next step is to develop interventions that can address population factors as well as individual behavior and risk. Community-based participatory research provides a platform for introducing context into intervention strategies as a component to be evaluated and not simply controlled. The community partnerships developed by the CPHHDs and shown in Table 1 ▶ are critical to the effective translation of research findings into applications in community settings. Partners provide important local knowledge, as well as access to political leaders, and these partnerships are the likely channels for dissemination of research findings about health disparities and for implementation of effective interventions.
Acknowledgments
This research was funded by the National Institute of Environmental Health Sciences (grants P50 ES012395, P50 ES012382, and P50 ES012383), the National Institute of Aging (grant P01 AG02323394), and the National Cancer Institute (grants P50 CA1065631, P50 CA015632, P50 CA106743, and P50 CA105641).
This article was originated by the leadership of the CPHHD program.
The authors acknowledge the editorial assistance of Lisa Kelly-Wilson at the Survey Research Laboratory, University of Illinois at Chicago.
Human Participant Protection No protocol approval was needed for this study.
Peer Reviewed
Contributors R. B. Warnecke was the lead author. A. Oh did the bibliographic work for the article and contributed to its content. N. Breen contributed to the conceptualization of the article and participated in writing the final draft. S. Gehlert contributed to the content of the article and assisted with writing. E. Paskett contributed to the content of the article and its preparation. K. L. Tucker, N. Lurie, T. Rebbeck, J. Goodwin, J. Flack, S. Srinivasan, and J. Kerner contributed to the content of the article. S. Heurtin-Roberts, R. Abeles, F. L. Tyson, and G. Patmios critically read drafts of the article. R. A. Hiatt critically read the article and provided important insights that guided its development.
References
- 1.Report of the Secretary’s Task Force on Black and Minority Health. Washington, DC: US Dept of Health and Human Services; 1985.
- 2.Stoto MA, Green, LW, Bailey LA, eds. Linking Research and Public Health Practice: A Review of CDC’s Program of Centers for Research and Demonstration of Health Promotion and Disease Prevention. Washington, DC: National Academy Press; 1997. [PubMed]
- 3.Healthy People 2010: Understanding and Improving Health. Washington, DC: US Dept of Health and Human Services; 2000.
- 4.Committee on the Review and Assessment of the NIH’s Strategic Research Plan and Budget to Reduce and Ultimately Eliminate Health Disparities. Examining the Health Disparities Research Plan of the National Institutes of Health: Unfinished Business. Washington, DC: National Academy Press; 2006.
- 5.Haynes MA, Smedley B, eds. The Unequal Burden of Cancer: An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved. Washington, DC: Institute of Medicine, National Academy Press; 1999. [PubMed]
- 6.Martin LG, Soldo BJ, eds. Racial and Ethnic Differences in the Health of Older Americans. Washington, DC: National Academy Press; 1997. [PubMed]
- 7.Anderson NB. Levels of analysis in health science: a framework for integrating sociobehavioral and biomedical research. Ann N Y Acad Sci. 1999:563–576. [DOI] [PubMed]
- 8.Office of Behavioral and Social Sciences Research. Toward Higher Levels of Analysis: Progress and Promise in Research on Social and Cultural Dimensions of Health. Bethesda, MD: National Institutes of Health; 2001. NIH publication 01-5020.
- 9.Singer BH, Ryff CD, eds.; Committee on Future Directions for Behavior and Social Sciences Research at the National Institutes of Health. New Horizons in Health: An Integrative Approach. Washington, DC: National Academies Press; 2001. [PubMed]
- 10.Smedley BD, Syme SL, eds. Promoting Health: Intervention Strategies From Social and Behavioral Research. Washington, DC: National Academy Press; 2000. [PubMed]
- 11.Hanna K, Coussens C. Rebuilding the Unity of Health and the Environment: A New Vision of Environmental Health for the 21st Century. Washington, DC: National Academies Press; 2001.
- 12.Institute of Medicine, Committee on Health and Behavior. Health and Behavior: The Interplay of Biological, Behavioral, and Societal Influences. Washington, DC: National Academies Press; 2001.
- 13.Stern PC, Carstensen LL, eds. The Aging Mind: Opportunities in Cognitive Research. Washington, DC: National Academy Press; 2000. [PubMed]
- 14.Finch CE, Vaupel JW, Kinsella K, eds. Cells and Surveys: Should Biological Measures Be Included in Social Science Research? Washington, DC: National Academies Press; 2001. [PubMed]
- 15.National Institutes of Health. Centers for Population Health and Health Disparities. Available at: http://grants2.nih.gov/grants/guide/rfa-files/RFA-ES-02-009.html. Accessed August 10, 2006.
- 16.AHRQ Focus on Research: Disparities in Health Care. Rockville, MD: Agency for Healthcare Research and Quality; 2002. AHRQ publication 02-M027. Available at: http://www.ahrq.gov/news/focus/disparhc.htm. Accessed August 28, 2007.
- 17.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14:32–38. [DOI] [PubMed] [Google Scholar]
- 18.Rose G. The Strategy of Prevention. New York, NY: Oxford Press; 1992.
- 19.Kindig DA. Understanding population health terminology. Milbank Q. 2007;85:139–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmot M, Wilkinson RG. Social Determinants of Health. 2nd ed. New York, NY: Oxford University Press; 2006.
- 21.Marmot M. The Status Syndrome: How Social Standing Affects Our Health and Longevity. New York, NY: Henry Holt; 2004.
- 22.Evans R, Barer M, Marmour T. Why Are Some People Healthy and Others Not? The Determinants of Health in Populations. New York, NY: Aldine de Gruyter; 1994.
- 23.Harper S, Lynch J. Methods for Measuring Cancer Disparities: Using Data Relevant to Healthy People 2010 Cancer-Related Objectives. Cancer Control Monograph Series, No. 6. Bethesda, MD: National Cancer Institute; 2005. NIH publication 05-5777.
- 24.Taylor SE, Repetti RL, Seeman T. Health psychology: what is an unhealthy environment and how does it get under the skin? Annu Rev Psychol. 1997; 48:411–417. [DOI] [PubMed] [Google Scholar]
- 25.Berkman LF, Glass T, Brissette I, Seeman TE. From social integration to health: Durkheim in the new millennium. Social Sci Med. 2000;51:843–857. [DOI] [PubMed] [Google Scholar]
- 26.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62:1650–1671. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian SV, Jones K, Duncan C. Multilevel methods for public health research. In: Kawachi I, Berkman LF, eds. Neighborhood and Health. New York, NY: Oxford University Press; 2003:65–111.
- 28.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995;Spec No:80–94. [PubMed]
- 29.Diez-Roux AV, Nieto GJ, Muntaner C, Tyroler HA, Comstock GW. Neighborhood environments and coronary heart disease: a multilevel analysis. Am J Epidemiol. 1997;146:48–63. [DOI] [PubMed] [Google Scholar]
- 30.Jones K, Duncan C. Individuals and their ecologies: analyzing the geography of chronic disease within a multilevel modeling framework. Health Place. 1995; 1:27–30. [Google Scholar]
- 31.Boardman JD, Saint Onge JM, Rogers RG, Denny JT. Race differentials in obesity: the impact of place. J Health Soc Behav. 2005;26:229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill TD, Ross CE, Angell RJ. Neighborhood disorder, psychophysiological distress, and health. J Health Soc Behav. 2005;46:140–186. [DOI] [PubMed] [Google Scholar]
- 33.Jenks C, Meyer SE. The social consequences of growing up in a poor neighborhood. In: Lynn LE, McGeary MGH, eds. Inner City Poverty in the United States. Washington DC: National Academy Press; 1990:111–186.
- 34.Raudenbush SW, Sampson RJ. Econometrics toward a science of assessing ecological settings with application to systematic social observation of neighborhoods. Sociol Methodol. 1999;29:1–41. [Google Scholar]
- 35.Ross CE, Mirowsky J. Neighborhood disadvantage, disorder and health. J Health Soc Behav. 2001;42:258–276. [PubMed] [Google Scholar]
- 36.Sampson RJ. How do communities undergird or undermine human development? Relevant contexts and social mechanisms. In: Booth A, Crouter N, eds. Does It Take a Village? Community Effects on Children, Adolescents, and Families. Mahwah, NJ: Lawrence Erlbaum; 2001:3–30.
- 37.Sampson RJ. Neighborhood level context and health: lessons from sociology. In: Kawachi I, Berkman LF, eds. Neighborhood and Health. New York, NY: Oxford University Press; 2003:132–146.
- 38.Heaney CA, Israel BA. Social networks and social support. In: Glanz K, Rimer BK, Lewis FM, eds. Health Behavior and Health Education: Theory, Research and Practice. San Francisco, CA: Jossey-Bass. 2002:185–209.
- 39.Kawachi I, Berkman LF. Social cohesion, social capital, and health. In: Berkman LF, Kawachi I, eds. Social Epidemiology. New York, NY: Oxford University Press; 2000:174–189.
- 40.Lochner KA, Kawchi I, Brennan RT, Buka SL. Social capital and neighborhood mortality rates in Chicago. Soc Sci Med. 2003;56:1797–1805. [DOI] [PubMed] [Google Scholar]
- 41.Seeman TE. Social ties and health: the benefits of social integration. Ann Epidemiol. 1996;5:442–451. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian SV, Lochner KA, Kawachi I. Neighborhood differences in social capital: a compositional artifact or a contextual construct? Health Place. 2003; 9:33–44. [DOI] [PubMed] [Google Scholar]
- 43.Szreter S, Woolcock M. Health by association? Social capital, social theory, and the political economy of public health. Int J Epidemiol. 2004;33:650–667. [DOI] [PubMed] [Google Scholar]
- 44.Thoits PA. Stress coping, and social support processes: where are we? What next? J Health Soc Behav. 1995;Spec No:53–79. [PubMed]
- 45.Fitzpatrick K, LaGory M. Unhealthy Places: The Ecology of Risk in the Urban Landscape. New York, NY: Routledge; 2000.
- 46.Sloggett A, Joshi H. Higher mortality in deprived areas: community or personal disadvantage? BMJ. 1994;309:1470–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waitzman NJ, Smith KR. Phantom of the area: poverty-area residence and mortality in the United States. Am J Pub Health. 1998;88:973–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Emmons KM. Health behaviors in a social context. In: Berkman L, Kawachi I, eds. Social Epidemiology. New York, NY: Oxford University Press; 2000:242–266.
- 49.Glanz K, Rimer BK, Lewis FM, eds. Health Behavior and Health Education: Theory, Research and Practice. San Francisco, CA: Jossey-Bass; 2002.
- 50.Baum A, Garafalo JP, Yali AM. Socioeconomic status and chronic stress. Ann N Y Acad Sci. 1999; 896:131–144. [DOI] [PubMed] [Google Scholar]
- 51.Cacioppo JT, Hawkley LC, Crawford LE, et al. Loneliness and health: potential mechanisms. Psychosom Med. 2002;64:407–414. [DOI] [PubMed] [Google Scholar]
- 52.Elliott M. The stress process in neighborhood context. Health Place. 2000;6:287–299. [DOI] [PubMed] [Google Scholar]
- 53.Falcon LM, Tucker KL. Prevalence and correlates of depressive symptomatology among Hispanic elders in Massachusetts. J Gerontol B Psychol Sci Soc Sci. 2000;55(2):S108–S116. [DOI] [PubMed] [Google Scholar]
- 54.Gallo LC, Matthews KA. Do negative emotions mediate the association between socioeconomic status and health? Ann N Y Acad Sci. 1999;896:226–245. [DOI] [PubMed] [Google Scholar]
- 55.McClintock MK, Conzen SD, Gehlert S, Masi C, Olopade F. Mammary cancer and social interactions: identifying multiple environments that regulate gene expression throughout the life span. J Gerontol B Psychol Sci Soc Sci. 2005;60(Spec No 1):32–41. [DOI] [PubMed] [Google Scholar]
- 56.Ryff CD, Singer B. From social structure to biology: integrative science in pursuit of human health and well-being. In: Snyder, CR, Lopez SJ, eds. Handbook of Positive Psychology. New York, NY: Oxford University Press; 2002.
- 57.Tucker KL, Bermudez O, Castanada C. Type 2 diabetes is prevalent and poorly controlled among His-panic elders. Am J Pub Health. 2000;90:1288–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hermes GL, Rosenthal L, Montag A, et al. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. Am J Physiol Regul Integr Comp Physiol. 2006;290:273–282. [DOI] [PubMed] [Google Scholar]
- 59.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. [DOI] [PubMed] [Google Scholar]
- 60.House JA, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. [DOI] [PubMed] [Google Scholar]
- 61.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–45. [DOI] [PubMed] [Google Scholar]
- 62.Eschbach K, Kuo YF, Goodwin JS. Ascertainment of Hispanic ethnicity on California death certificates: implications for the explanation of the Hispanic mortality advantage. Am J Pub Health. 2006;96:2209–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eschbach K, Stimpson JP, Kuo YF, et al. Mortality of Hispanic immigrants and US-born Hispanics at younger ages: a re-examination of recent patterns. Am J Pub Health. 2007;97(7):1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peek MK, Cutchin MP, Freeman DH, et al. Perceived health change in the aftermath of a petrochemical accident: an examination of pre-disaster, within-disaster, and post-disaster variables. J Epidemiol Community Health. 2008;62(2):106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cutchin MP, Martin KR, Owen SV, et al. Concerns about petrochemical health risk before and after a refinery explosion. Risk Anal. In press. [DOI] [PMC free article] [PubMed]
- 66.Barrett RE, Cho YI, Weaver KE, et al. Neighborhood change and distant metastasis at diagnosis of breast cancer. Ann Epidemiol. 2008;18:43–47. [DOI] [PubMed] [Google Scholar]
- 67.Tucker KL, Falcon LM, Bianchi LI, Cacho E, Bermudez OI. Self reported prevalence and health correlates of functional limitation among Massachusetts elderly Puerto Ricans, Dominicans, and a non-Hispanic white neighborhood comparison group. J Gerontol A Biol Sci Med Sci. 2000;55(2):M90–M97. [DOI] [PubMed] [Google Scholar]
- 68.Zeigler-Johnson CM, Friebel T, Walker AH, et al. CYP3A4, CYP3A5, and CYP3A43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer Res. 2004;64:8461–8467. [DOI] [PubMed] [Google Scholar]
- 69.Division of Strategic Coordination, Office of Portfolio Analysis and Strategic Initiatives, National Institutes of Health. NIH Roadmap for Medical Research. Available at: http://nihroadmap.nih.gov. Accessed September 11, 2006.