Abstract
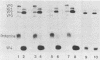
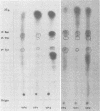
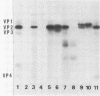
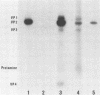
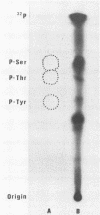
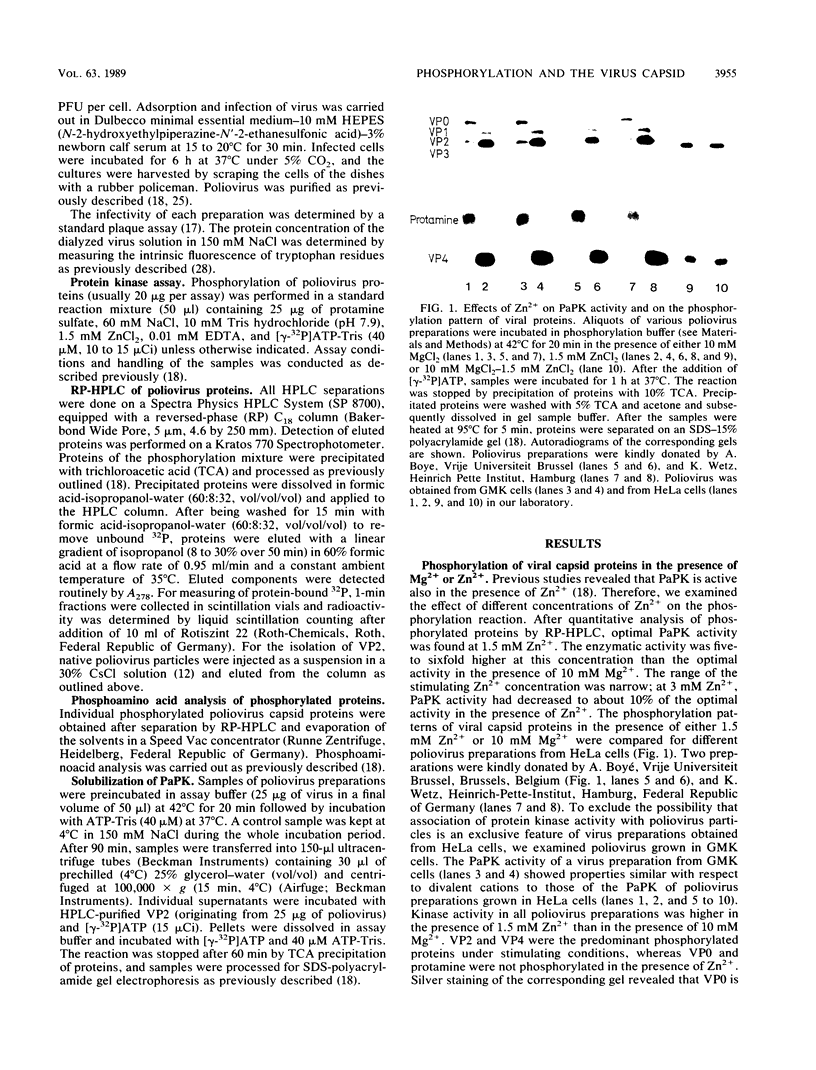
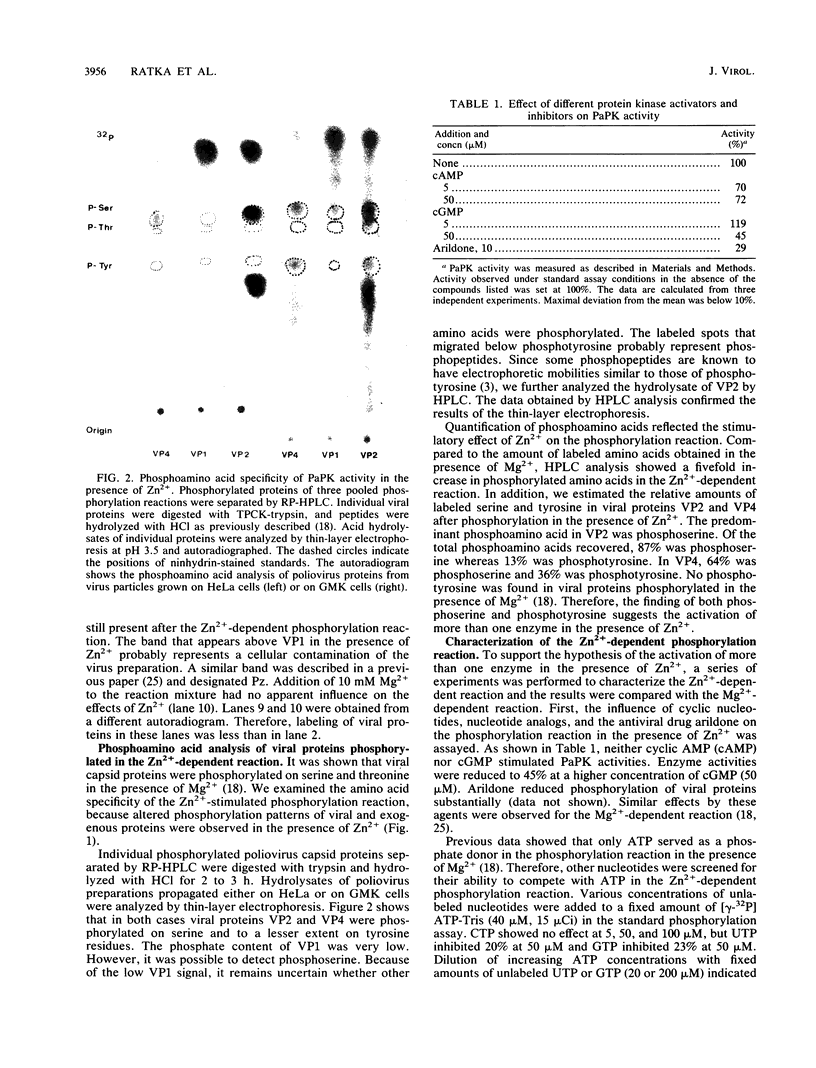
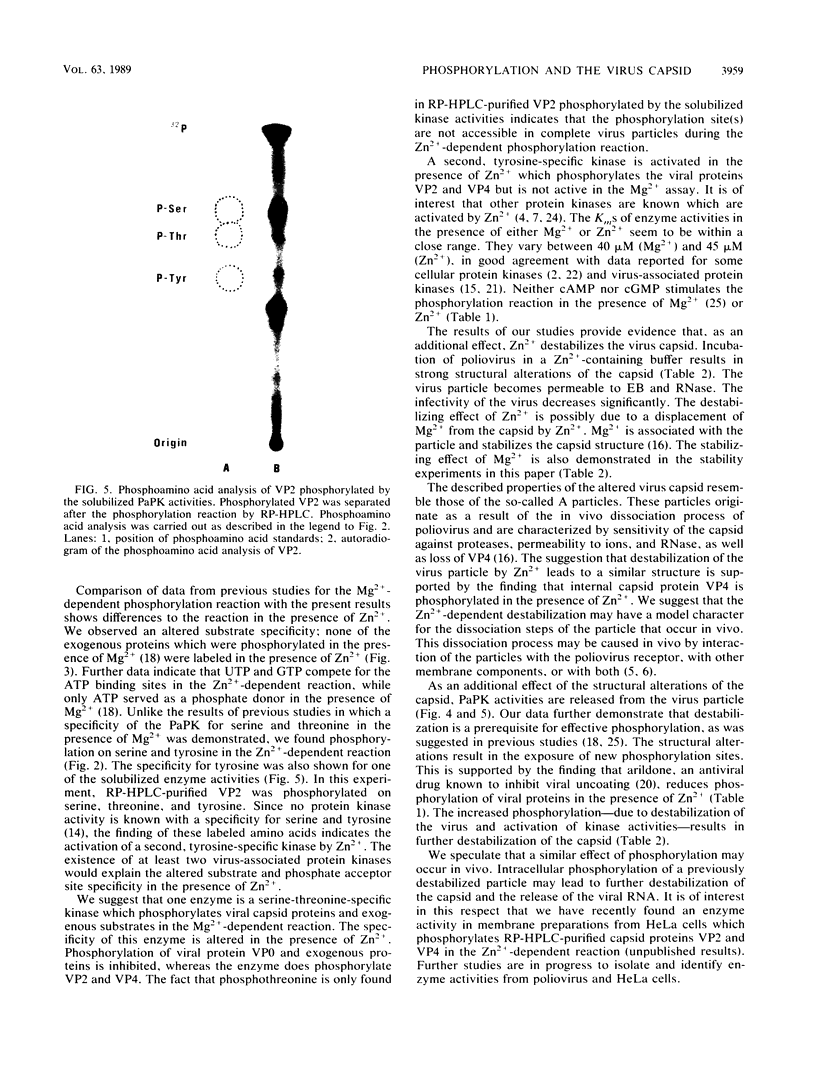
The previously described poliovirus-associated protein kinase activity phosphorylates viral proteins VP0 and VP2 as well as exogenous proteins in the presence of Mg2+. In this paper, the effect of Zn2+ on the phosphorylation reaction and the stability of the poliovirus capsid has been studied in detail and compared to that of Mg2+. Phosphorylation patterns of viral and other proteins depend on the divalent cation present. In the presence of Zn2+, phosphorylation of capsid proteins VP2 and VP4 is significantly higher while phosphorylation of VP0 and exogenous phosphate acceptor proteins is not detected. Our results indicate the activation of more than one virus-associated protein kinase by Zn2+. The ion-dependent behavior of the enzyme activities is observed independently of whether the virus was obtained from HeLa or green monkey kidney cells. The poliovirus capsid is destabilized by Zn2+. The destabilization leads to a substantially increased permeability of virus particles to ethidium bromide and RNase, concomitant with decreased infectivity of the sample. This alteration of the poliovirus capsid structure is a prerequisite for effective phosphorylation of viral capsid proteins. The increased level of phosphorylation of viral capsid proteins results in further destabilization of the viral capsid. As a result of the conformational changes, poliovirus-associated protein kinase activities dissociate from the virus particle. High-performance liquid chromatography-purified viral protein VP2 is phosphorylated by the released enzymes on serine, threonine, and tyrosine in the presence of Zn2+. We suggest that the destabilizing effect of phosphorylation on the viral capsid plays a role in uncoating of poliovirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cajean-Feroldi C., Loeb J., Meguenni S., Girard M. Protein kinases associated with the adenovirus single-stranded DNA-binding protein. Eur J Biochem. 1981 Nov;120(1):79–87. doi: 10.1111/j.1432-1033.1981.tb05672.x. [DOI] [PubMed] [Google Scholar]
- Cobb M. H., Rosen O. M. The insulin receptor and tyrosine protein kinase activity. Biochim Biophys Acta. 1984;738(1-2):1–8. doi: 10.1016/0304-419x(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Csermely P., Szamel M., Resch K., Somogyi J. Zinc can increase the activity of protein kinase C and contributes to its binding to plasma membranes in T lymphocytes. J Biol Chem. 1988 May 15;263(14):6487–6490. [PubMed] [Google Scholar]
- De Sena J., Mandel B. Studies on the in vitro uncoating of poliovirus. I. Characterization of the modifying factor and the modifying reaction. Virology. 1976 Apr;70(2):470–483. doi: 10.1016/0042-6822(76)90288-9. [DOI] [PubMed] [Google Scholar]
- De Sena J., Mandel B. Studies on the in vitro uncoating of poliovirus. II. Characteristics of the membrane-modified particle. Virology. 1977 May 15;78(2):554–566. doi: 10.1016/0042-6822(77)90130-1. [DOI] [PubMed] [Google Scholar]
- Findik D., Presek P. Zn2+ enhances protein tyrosine kinase activity of human platelet membranes. FEBS Lett. 1988 Aug 1;235(1-2):51–56. doi: 10.1016/0014-5793(88)81232-8. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Goldmann U., Müller R., Stibbe W., Wolff W. Specificity and localization of the hepatitis B virus-associated protein kinase. J Virol. 1982 Jun;42(3):761–766. doi: 10.1128/jvi.42.3.761-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmel M., Zibirre R., Koch G. Fluorescence spectrophotometric study of structural alterations in the capsid of poliovirus. Arch Virol. 1983;78(3-4):191–201. doi: 10.1007/BF01311314. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Baxt B., La Torre J. L., Bachrach H. L. Identification of a protein kinase activity in purified foot- and-mouth disease virus. J Virol. 1981 Aug;39(2):455–462. doi: 10.1128/jvi.39.2.455-462.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman N., Baltimore D. A plasma membrane component able to bind and alter virions of poliovirus type 1: studies on cell-free alteration using a simplified assay. Virology. 1977 Oct 1;82(1):25–36. doi: 10.1016/0042-6822(77)90029-0. [DOI] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Rapid analytical and preparative separation of structural polypeptides of poliovirus by reverse-phase high-performance liquid chromatography. J Virol Methods. 1983 May;6(5):283–293. doi: 10.1016/0166-0934(83)90043-5. [DOI] [PubMed] [Google Scholar]
- Howard C. R., Buchmeier M. J. A protein kinase activity in lymphocytic choriomeningitis virus and identification of the phosphorylated product using monoclonal antibody. Virology. 1983 Apr 30;126(2):538–547. doi: 10.1016/s0042-6822(83)80011-7. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Quintrell N., Bishop J. M. An agar cell-suspension plaque assay for isolated viral RNA. Biochem Biophys Res Commun. 1966 Aug 12;24(3):304–309. doi: 10.1016/0006-291x(66)90155-0. [DOI] [PubMed] [Google Scholar]
- Lackmann M., Ueckermann C., Engelmann K., Koch G. Properties of poliovirus associated protein kinase. Arch Virol. 1987;95(1-2):1–16. doi: 10.1007/BF01311330. [DOI] [PubMed] [Google Scholar]
- Lemaster S., Roizman B. Herpes simplex virus phosphoproteins. II. Characterization of the virion protein kinase and of the polypeptides phosphorylated in the virion. J Virol. 1980 Sep;35(3):798–811. doi: 10.1128/jvi.35.3.798-811.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Caliguiri L. A., Eggers H. J. Inhibition of uncoating of poliovirus by arildone, a new antiviral drug. Virology. 1979 Sep;97(2):307–315. doi: 10.1016/0042-6822(79)90342-8. [DOI] [PubMed] [Google Scholar]
- Michelson S., Tardy-Panit M., Bârzu O. Catalytic properties of a human cytomegalovirus-induced protein kinase. Eur J Biochem. 1985 Jun 3;149(2):393–399. doi: 10.1111/j.1432-1033.1985.tb08938.x. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Kuenzel E. A., Casnellie J. E., Krebs E. G. A comparison of the insulin- and epidermal growth factor-stimulated protein kinases from human placenta. J Biol Chem. 1984 Aug 10;259(15):9913–9921. [PubMed] [Google Scholar]
- Roby C., Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986 Sep;59(3):714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakihara Y., Volpe J. J. Zn2+, not Ca2+, is the most effective cation for activation of dolichol kinase of mammalian brain. J Biol Chem. 1985 Dec 15;260(29):15413–15419. [PubMed] [Google Scholar]
- Schärli C. E., Koch G. Protein kinase activity in purified poliovirus particles and empty viral capsid preparations. J Gen Virol. 1984 Jan;65(Pt 1):129–139. doi: 10.1099/0022-1317-65-1-129. [DOI] [PubMed] [Google Scholar]
- Stevely W. S., Katan M., Stirling V., Smith G., Leader D. P. Protein kinase activities associated with the virions of pseudorabies and herpes simplex virus. J Gen Virol. 1985 Apr;66(Pt 4):661–673. doi: 10.1099/0022-1317-66-4-661. [DOI] [PubMed] [Google Scholar]
- Tsuzuki J., Luftig R. B. Evidence for the ubiquitous presence of a protein kinase in human adenoviruses capable of preferentially phosphorylating capsid protein IIIa. Intervirology. 1985;23(2):90–96. doi: 10.1159/000149590. [DOI] [PubMed] [Google Scholar]
- Vilereal M. L., Cook J. S. Role of the membrane potential in serum-stimulated uptake of amino acid in a diploid human fibroblast. J Supramol Struct. 1977;6(2):179–189. doi: 10.1002/jss.400060204. [DOI] [PubMed] [Google Scholar]