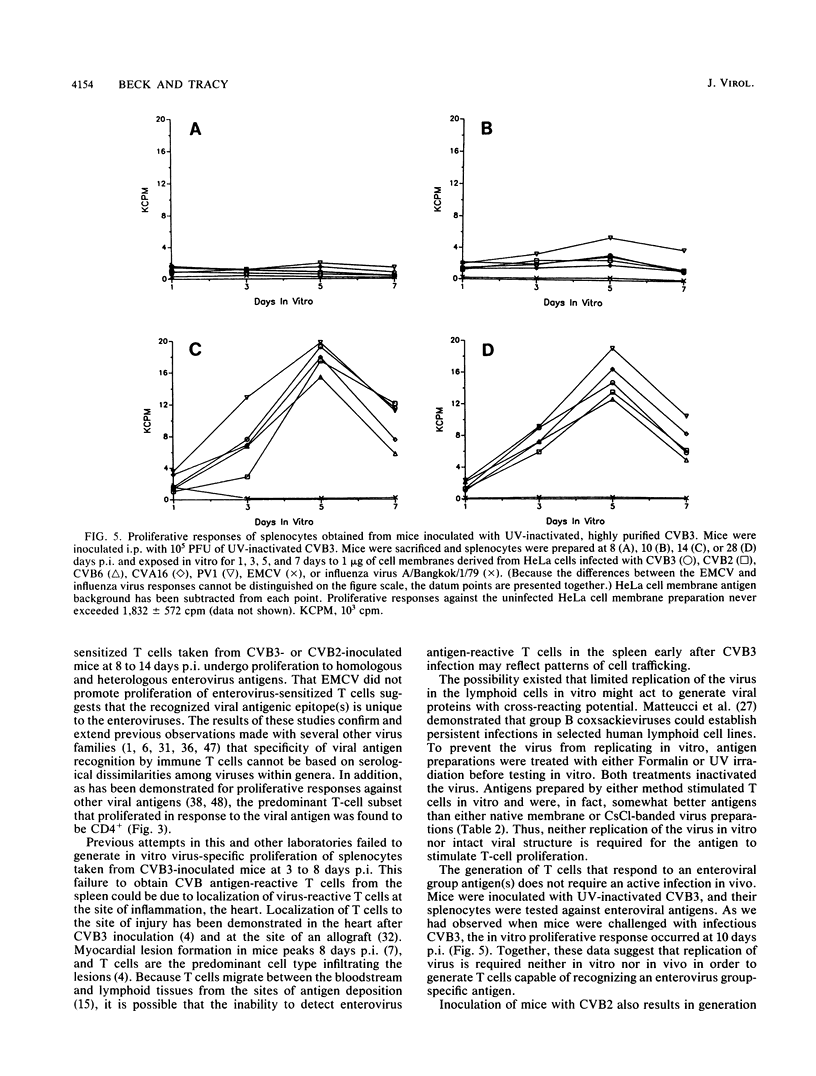
Abstract
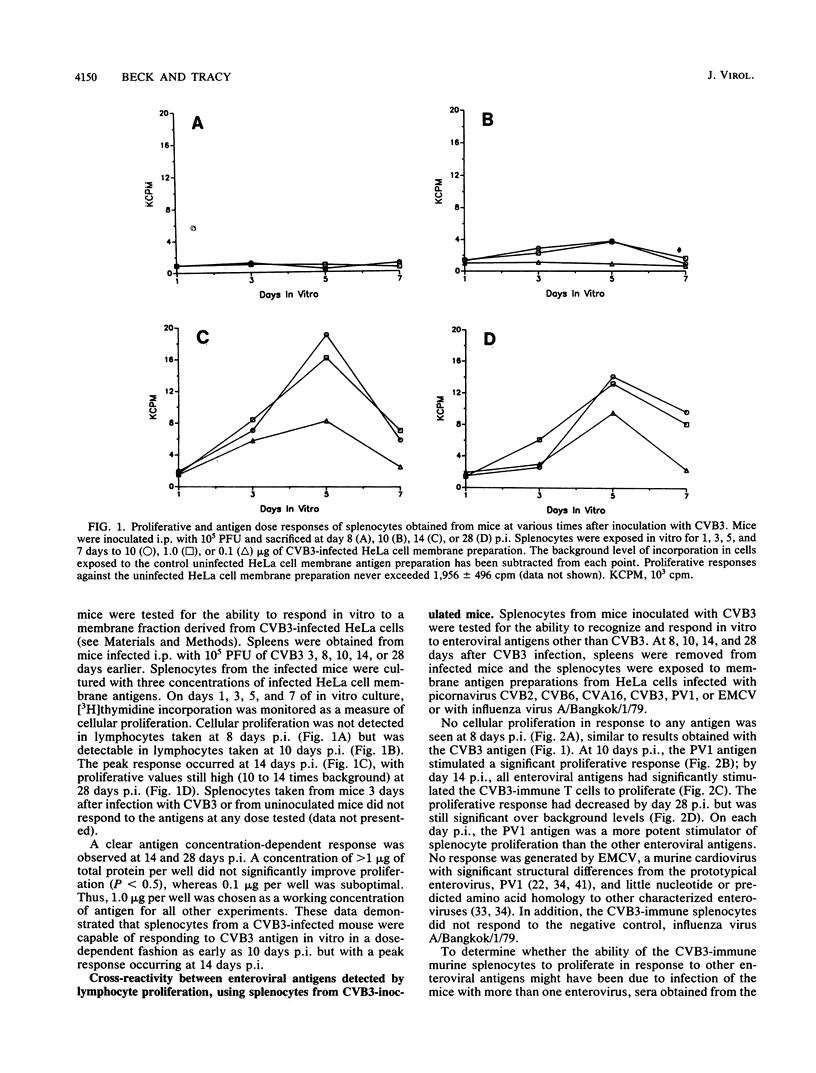
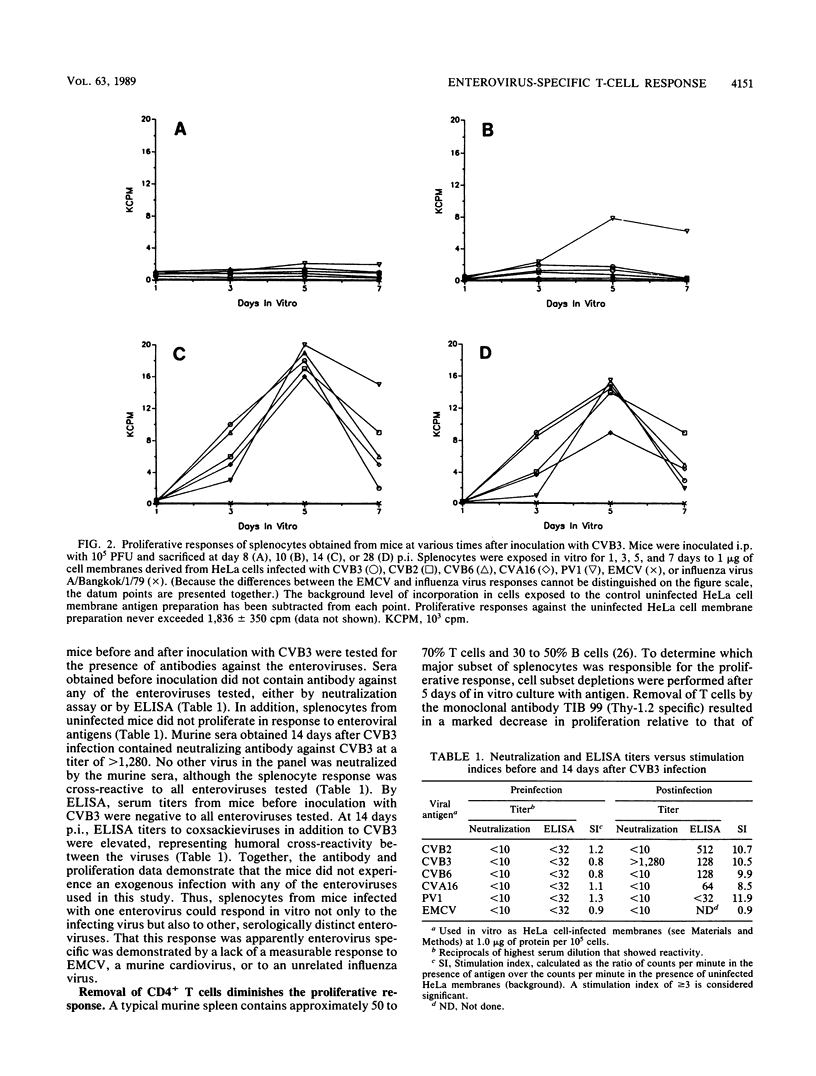
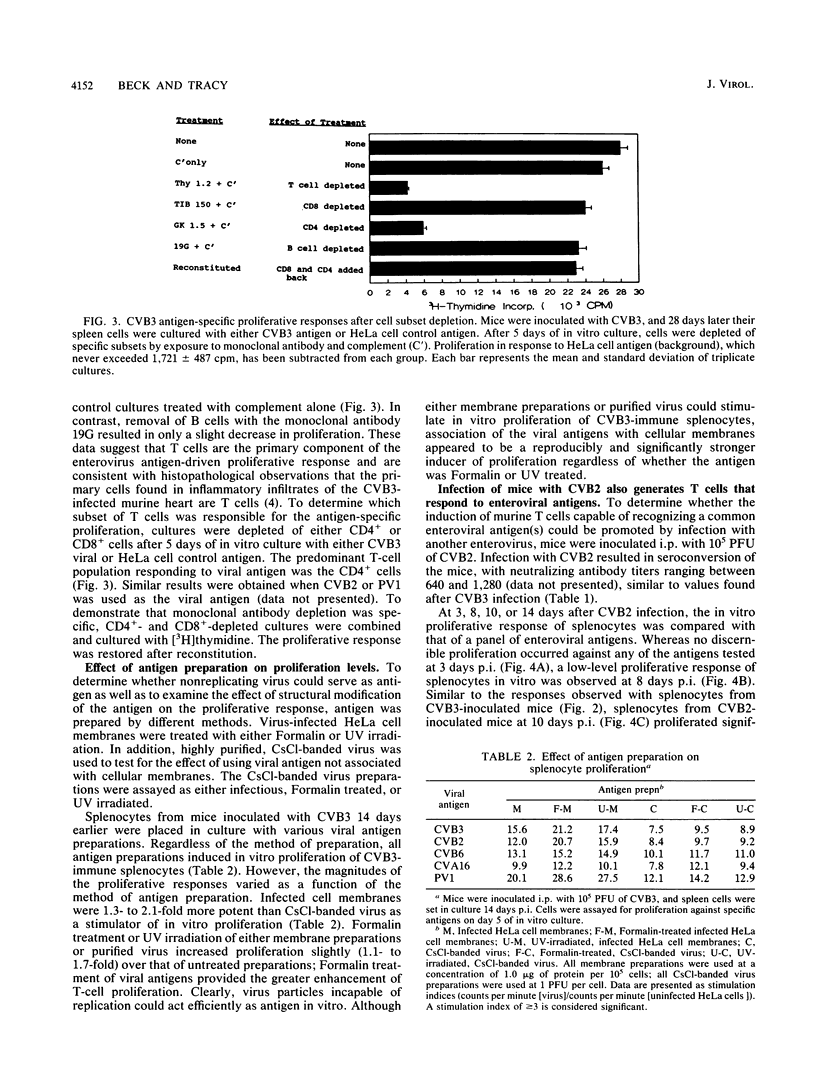
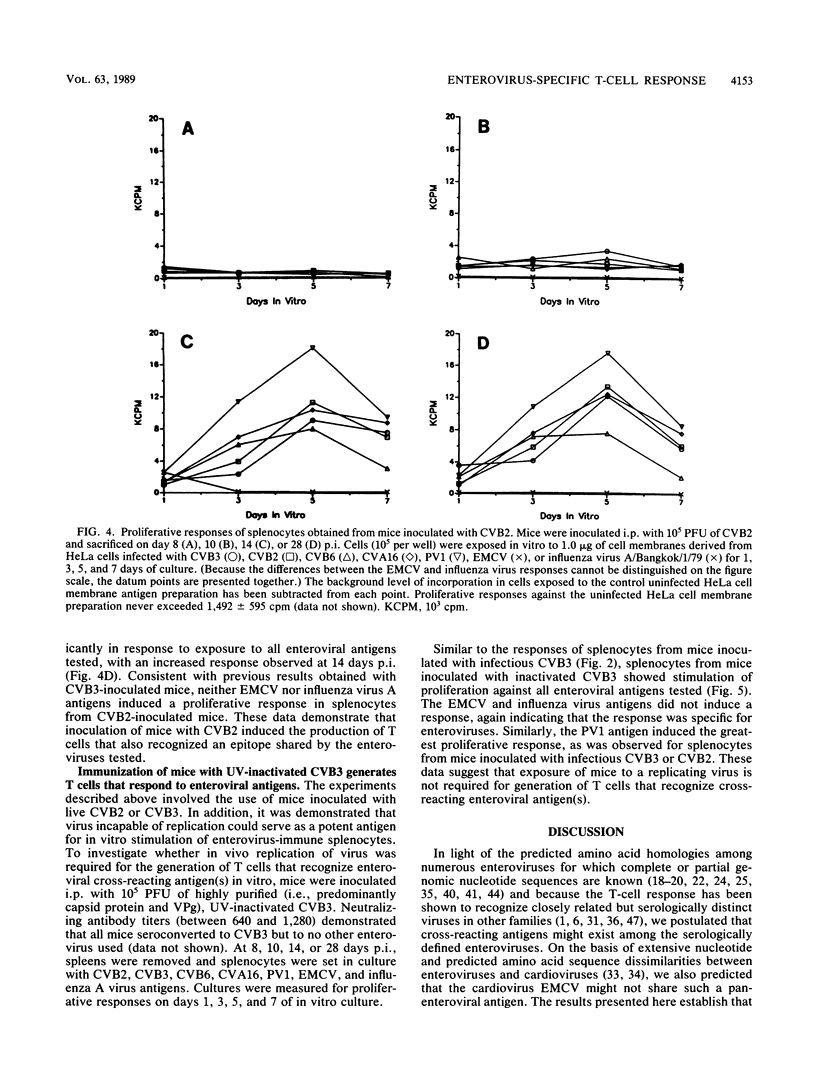
Splenocytes taken from mice inoculated with coxsackievirus B3 (CVB3) (Nancy) developed an in vitro proliferative response against CVB3 antigen. This response could not be detected earlier than 8 days postinoculation but could be detected up to 28 days after exposure to CB3. CVB3-sensitized splenocytes responded not only to the CVB3 antigen but to other enteroviruses as well. This response was found to be enterovirus specific in that no response was detected to a non-enteroviral picornavirus, encephalomyocarditis virus, or to an unrelated influenza virus. The generation of a splenocyte population capable of responding to an enterovirus group antigen(s) was not limited to inoculation of mice with CVB3, as similar responses were generated when mice were inoculated with CVB2. Cell subset depletions revealed that the major cell type responding to the enterovirus group antigen(s) was the CD4+ T cell. Current evidence suggests that the group antigen(s) resides in the structural proteins of the virus, since spleen cells from mice inoculated with a UV-inactivated, highly purified preparation of CVB3 virions also responded in vitro against enteroviral antigens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. I. Generation of a virus-strain specific and a cross-reactive subpopulation of cytotoxic T cells in the response to type A influenza viruses of different subtypes. Cell Immunol. 1977 Oct;33(2):423–436. doi: 10.1016/0008-8749(77)90170-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Karunas R. S. Relationship of congenital anomalies and maternal infection with selected enteroviruses. Am J Epidemiol. 1972 Mar;95(3):207–217. doi: 10.1093/oxfordjournals.aje.a121388. [DOI] [PubMed] [Google Scholar]
- Deguchi H., Kitaura Y., Morita H., Kotaka M., Kawamura K. Cell-mediated immunity in Coxsackie B3 virus myocarditis in mice--in situ characterization by monoclonal antibody of mononuclear cell infiltrates. Heart Vessels Suppl. 1985;1:221–227. doi: 10.1007/BF02072397. [DOI] [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Gomez P. T., Duffey P. S., Grant J. A., Trent D. W., Witherspoon S. M., Paque R. E. Characterization and myocarditic capabilities of coxsackievirus B3 variants in selected mouse strains. J Virol. 1984 Nov;52(2):598–605. doi: 10.1128/jvi.52.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., LaBadie D. R., Paque R. E., Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3(3):207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- Grist N. R., Bell E. J., Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114–157. [PubMed] [Google Scholar]
- Hashimoto I., Komatsu T. Myocardial changes after infection with Coxsackie virus B3 in nude mice. Br J Exp Pathol. 1978 Feb;59(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Hopt U. T., Sullivan W., Hoffman R., Simmons R. L. Migration and cell recruiting activity of specifically sensitized lymphocytes in sponge matrix allografts. Transplantation. 1980 Dec;30(6):411–416. doi: 10.1097/00007890-198012000-00005. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Iizuka N., Kuge S., Nomoto A. Complete nucleotide sequence of the genome of coxsackievirus B1. Virology. 1987 Jan;156(1):64–73. doi: 10.1016/0042-6822(87)90436-3. [DOI] [PubMed] [Google Scholar]
- Jenkins O., Booth J. D., Minor P. D., Almond J. W. The complete nucleotide sequence of coxsackievirus B4 and its comparison to other members of the Picornaviridae. J Gen Virol. 1987 Jul;68(Pt 7):1835–1848. doi: 10.1099/0022-1317-68-7-1835. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto C., Misaki T., Crumpacker C. S., Abelmann W. H. Serial immunologic identification of lymphocyte subsets in murine coxsackievirus B3 myocarditis: different kinetics and significance of lymphocyte subsets in the heart and in peripheral blood. Circulation. 1988 Mar;77(3):645–653. doi: 10.1161/01.cir.77.3.645. [DOI] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- La Monica N., Meriam C., Racaniello V. R. Mapping of sequences required for mouse neurovirulence of poliovirus type 2 Lansing. J Virol. 1986 Feb;57(2):515–525. doi: 10.1128/jvi.57.2.515-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg A. M., Stålhandske P. O., Pettersson U. Genome of coxsackievirus B3. Virology. 1987 Jan;156(1):50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Loor F., Kindred B. Differentiation of T-cell precursors in nude mice demonstrated by immunofluorescence of T-cell membrane markers. J Exp Med. 1973 Nov 1;138(5):1044–1055. doi: 10.1084/jem.138.5.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteucci D., Paglianti M., Giangregorio A. M., Capobianchi M. R., Dianzani F., Bendinelli M. Group B coxsackieviruses readily establish persistent infections in human lymphoid cell lines. J Virol. 1985 Nov;56(2):651–654. doi: 10.1128/jvi.56.2.651-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus B. M., Gauntt C. J., Cassling R. S. Immunopathologic basis of myocardial injury. Cardiovasc Clin. 1988;18(2):163–184. [PubMed] [Google Scholar]
- Melnick J. L. Enterovirus type 71 infections: a varied clinical pattern sometimes mimicking paralytic poliomyelitis. Rev Infect Dis. 1984 May-Jun;6 (Suppl 2):S387–S390. doi: 10.1093/clinids/6.supplement_2.s387. [DOI] [PubMed] [Google Scholar]
- Mullbacher A., Marshall I. D., Blanden R. V. Cross-reactive cytotoxic T cells to alphavirus infection. Scand J Immunol. 1979;10(4):291–296. doi: 10.1111/j.1365-3083.1979.tb01353.x. [DOI] [PubMed] [Google Scholar]
- Orosz C. G., Zinn N. E., Sirinek L., Ferguson R. M. In vivo mechanisms of alloreactivity. I. Frequency of donor-reactive cytotoxic T lymphocytes in sponge matrix allografts. Transplantation. 1986 Jan;41(1):75–83. [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Cross-reactive cytotoxic T cells to serologically distinct vesicular stomatitis virus. J Immunol. 1980 May;124(5):2301–2308. [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rothman A. L., Kurane I., Zhang Y. M., Lai C. J., Ennis F. A. Dengue virus-specific murine T-lymphocyte proliferation: serotype specificity and response to recombinant viral proteins. J Virol. 1989 Jun;63(6):2486–2491. doi: 10.1128/jvi.63.6.2486-2491.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Reeve P., Minor P. D., Schild G. C., Almond J. W. Comparison of the complete nucleotide sequences of the genomes of the neurovirulent poliovirus P3/Leon/37 and its attenuated Sabin vaccine derivative P3/Leon 12a1b. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1539–1543. doi: 10.1073/pnas.81.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Kohara M., Kataoka Y., Suganuma T., Omata T., Imura N., Nomoto A. Complete nucleotide sequences of all three poliovirus serotype genomes. Implication for genetic relationship, gene function and antigenic determinants. J Mol Biol. 1984 Apr 25;174(4):561–585. doi: 10.1016/0022-2836(84)90084-6. [DOI] [PubMed] [Google Scholar]
- Tracy S., Chapman N. M., Liu H. L. Molecular cloning and partial characterization of the coxsackievirus B3 genome. Brief report. Arch Virol. 1985;85(1-2):157–163. doi: 10.1007/BF01317016. [DOI] [PubMed] [Google Scholar]
- Tracy S., Liu H. L., Chapman N. M. Coxsackievirus B3: primary structure of the 5' non-coding and capsid protein-coding regions of the genome. Virus Res. 1985 Oct;3(3):263–270. doi: 10.1016/0168-1702(85)90050-4. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Paque R. E., Gauntt C. J. Isolation of Coxsackievirus B3 temperture-sensitive mutants and their assignment to complementation groups. Biochem Biophys Res Commun. 1976 May 23;76(2):368–375. doi: 10.1016/0006-291x(77)90734-3. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Wang K. G., Sun L. Z., Jubelt B., Waltenbaugh C. Cell-mediated immune responses to poliovirus. I. Conditions for induction, characterization of effector cells, and cross-reactivity between serotypes for delayed hypersensitivity and T cell proliferative responses. Cell Immunol. 1989 Apr 1;119(2):252–262. doi: 10.1016/0008-8749(89)90242-6. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- el-Khatib M. R., Chason J. L., Ho K. L., Silberberg B., Lerner A. M. Coxsackievirus B4 myocarditis in mice: valvular changes in virus-infected and control animals. J Infect Dis. 1978 Apr;137(4):410–420. doi: 10.1093/infdis/137.4.410. [DOI] [PubMed] [Google Scholar]


