Abstract
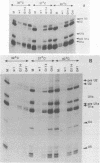
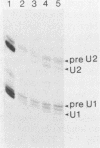
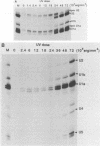
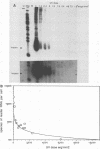
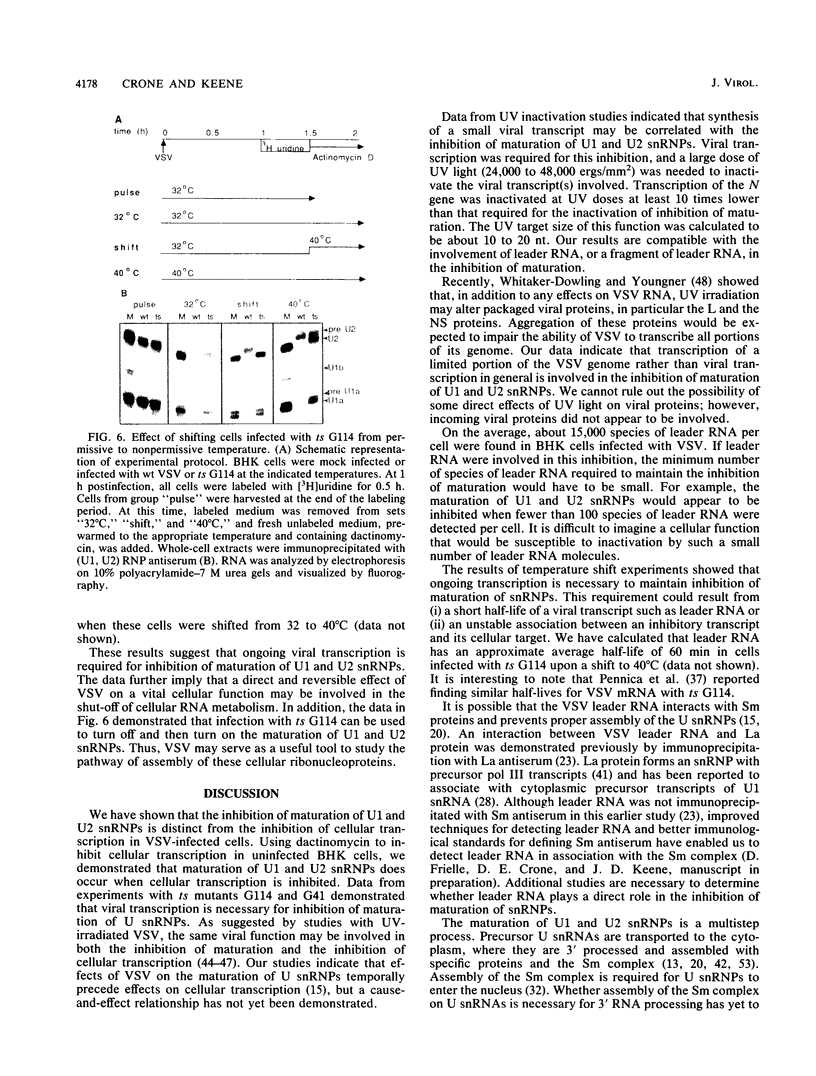
Infection of baby hamster kidney cells with vesicular stomatitis virus (VSV) results in the accumulation of immature U1 and U2 small nuclear ribonucleoproteins (snRNPs) that contain precursor U RNAs and at least some of the proteins specific for U1 and U2 snRNAs but lack the Sm complex of proteins that is common to these U snRNAs. The VSV function required for this effect is not known, but direct inhibition of cellular transcription did not alter the maturation of U1 and U2 snRNPs. On the other hand, viral transcription but not viral translation was required to inhibit U1 and U2 snRNP maturation. Temperature shift experiments with the mutant G114 showed that ongoing viral transcription was necessary, but that viral mRNA was not required for this inhibition. Furthermore, the VSV function involved in the inhibition of maturation of U1 and U2 snRNPs had a small UV target size of approximately 10 to 20 nucleotides. We demonstrate that temperature-sensitive mutants of VSV can be used as a tool to initiate the assembly of snRNPs in infected cells. These results are compatible with the suggestion that perturbation of snRNP metabolism by VSV precedes and is distinct from the effect of VSV on cellular RNA synthesis, although VSV leader RNA may be involved in both these functions.
Full text
PDF

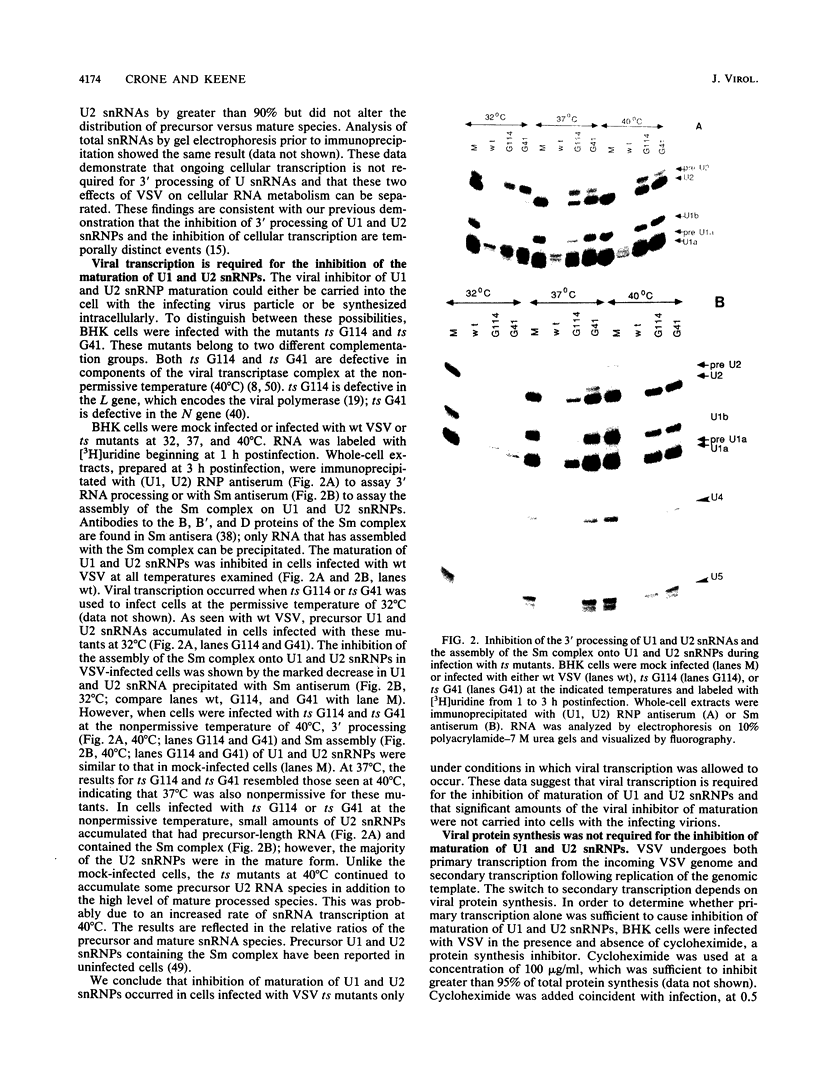



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Bringmann P., Lührmann R. Purification of the individual snRNPs U1, U2, U5 and U4/U6 from HeLa cells and characterization of their protein constituents. EMBO J. 1986 Dec 20;5(13):3509–3516. doi: 10.1002/j.1460-2075.1986.tb04676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Combard A., Martinet C., Printz Ane C., Friedman A., Printz P. Transcription and replication of vesicular stomatitis virus: effects of temperature-sensitive mutations in complementation group IV. J Virol. 1974 Apr;13(4):922–930. doi: 10.1128/jvi.13.4.922-930.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunigan D. D., Baird S., Lucas-Lenard J. Lack of correlation between the accumulation of plus-strand leader RNA and the inhibition of protein and RNA synthesis in vesicular stomatitis virus infected mouse L cells. Virology. 1986 Apr 15;150(1):231–246. doi: 10.1016/0042-6822(86)90282-5. [DOI] [PubMed] [Google Scholar]
- Dunigan D. D., Lucas-Lenard J. M. Two transcription products of the vesicular stomatitis virus genome may control L-cell protein synthesis. J Virol. 1983 Feb;45(2):618–626. doi: 10.1128/jvi.45.2.618-626.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri G. L., Sayavedra M. S. Small RNAs in the nucleus and cytoplasm of HeLa cells. Biochem Biophys Res Commun. 1976 Sep 20;72(2):507–512. doi: 10.1016/s0006-291x(76)80070-8. [DOI] [PubMed] [Google Scholar]
- Fisher D. E., Conner G. E., Reeves W. H., Blobel G., Kunkel H. G. Synthesis and assembly of human small nuclear ribonucleoproteins generated by cell-free translation. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6356–6360. doi: 10.1073/pnas.80.20.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. E., Conner G. E., Reeves W. H., Wisniewolski R., Blobel G. Small nuclear ribonucleoprotein particle assembly in vivo: demonstration of a 6S RNA-free core precursor and posttranslational modification. Cell. 1985 Oct;42(3):751–758. doi: 10.1016/0092-8674(85)90271-5. [DOI] [PubMed] [Google Scholar]
- Fisher D. E., Reeves W. H., Conner G. E., Blobel G., Kunkel H. G. Pulse labeling of small nuclear ribonucleoproteins in vivo reveals distinct patterns of antigen recognition by human autoimmune antibodies. Proc Natl Acad Sci U S A. 1984 May;81(10):3185–3189. doi: 10.1073/pnas.81.10.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco L. D., Kurilla M. G., Keene J. D. Rapid inhibition of processing and assembly of small nuclear ribonucleoproteins after infection with vesicular stomatitis virus. Mol Cell Biol. 1987 Mar;7(3):1148–1155. doi: 10.1128/mcb.7.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski P. J., Seiler S. R., Sharp P. A. A multicomponent complex is involved in the splicing of messenger RNA precursors. Cell. 1985 Aug;42(1):345–353. doi: 10.1016/s0092-8674(85)80130-6. [DOI] [PubMed] [Google Scholar]
- Grinnell B. W., Wagner R. R. Comparative inhibition of cellular transcription by vesicular stomatitis virus serotypes New Jersey and Indiana: role of each viral leader RNA. J Virol. 1983 Oct;48(1):88–101. doi: 10.1128/jvi.48.1.88-101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger M., Pettersson I., Steitz J. A. Isolation of small nuclear ribonucleoproteins containing U1, U2, U4, U5, and U6 RNAs. J Biol Chem. 1983 Feb 25;258(4):2604–2613. [PubMed] [Google Scholar]
- Hunt D. M., Wagner R. R. Location of the transcription defect in group I temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):28–35. doi: 10.1128/jvi.13.1.28-35.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D. Interactions between small viral RNAs of vesicular stomatitis virus and components of cellular gene expression. Microbiol Sci. 1985 May;2(5):152–156. [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A., Rosenberg M. Nucleotide sequence homology at the 3' termini of RNA from vesicular stomatitis virus and its defective interfering particles. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3225–3229. doi: 10.1073/pnas.75.7.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Keene J. D. The leader RNA of vesicular stomatitis virus is bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1983 Oct;34(3):837–845. doi: 10.1016/0092-8674(83)90541-x. [DOI] [PubMed] [Google Scholar]
- Kurilla M. G., Piwnica-Worms H., Keene J. D. Rapid and transient localization of the leader RNA of vesicular stomatitis virus in the nuclei of infected cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5240–5244. doi: 10.1073/pnas.79.17.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Liautard J. P., Sri-Widada J., Brunel C., Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells. Proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982 Dec 15;162(3):623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Eukaryotic small ribonucleoproteins. Anti-La human autoantibodies react with U1 RNA-protein complexes. J Biol Chem. 1984 Feb 10;259(3):1929–1933. [PubMed] [Google Scholar]
- Madore S. J., Wieben E. D., Pederson T. Intracellular site of U1 small nuclear RNA processing and ribonucleoprotein assembly. J Cell Biol. 1984 Jan;98(1):188–192. doi: 10.1083/jcb.98.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., De Robertis E. M. Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell. 1985 Jan;40(1):111–118. doi: 10.1016/0092-8674(85)90314-9. [DOI] [PubMed] [Google Scholar]
- McGowan J. J., Emerson S. U., Wagner R. R. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell. 1982 Feb;28(2):325–333. doi: 10.1016/0092-8674(82)90350-6. [DOI] [PubMed] [Google Scholar]
- Mimori T., Hinterberger M., Pettersson I., Steitz J. A. Autoantibodies to the U2 small nuclear ribonucleoprotein in a patient with scleroderma-polymyositis overlap syndrome. J Biol Chem. 1984 Jan 10;259(1):560–565. [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Mount S. M., Steitz J. A., Sharp P. A. Splicing of messenger RNA precursors is inhibited by antisera to small nuclear ribonucleoprotein. Cell. 1983 Nov;35(1):101–107. doi: 10.1016/0092-8674(83)90212-x. [DOI] [PubMed] [Google Scholar]
- Pennica D., Lynch K. R., Cohen P. S., Ennis H. L. Decay of vesicular stomatitis virus mRNAs in vivo. Virology. 1979 Apr 30;94(2):484–487. doi: 10.1016/0042-6822(79)90480-x. [DOI] [PubMed] [Google Scholar]
- Pettersson I., Hinterberger M., Mimori T., Gottlieb E., Steitz J. A. The structure of mammalian small nuclear ribonucleoproteins. Identification of multiple protein components reactive with anti-(U1)ribonucleoprotein and anti-Sm autoantibodies. J Biol Chem. 1984 May 10;259(9):5907–5914. [PubMed] [Google Scholar]
- Piwnica-Worms H., Keene J. D. Replication of the vesicular stomatitis virus genome in permissive and nonpermissive host cells. J Biol Chem. 1985 Sep 5;260(19):10503–10511. [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Sauterer R. A., Feeney R. J., Zieve G. W. Cytoplasmic assembly of snRNP particles from stored proteins and newly transcribed snRNA's in L929 mouse fibroblasts. Exp Cell Res. 1988 Jun;176(2):344–359. doi: 10.1016/0014-4827(88)90336-9. [DOI] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Carroll A. R., Shattuck D. M., Wagner R. R. Use of UV irradiation to identify the genetic information of vesicular stomatitis virus responsible for shutting off cellular RNA synthesis. J Virol. 1979 Jun;30(3):746–753. doi: 10.1128/jvi.30.3.746-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Inhibition of RNA synthesis in mouse myeloma cells infected with vesicular stomatitis virus. J Virol. 1978 Mar;25(3):770–780. doi: 10.1128/jvi.25.3.770-780.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weck P. K., Wagner R. R. Transcription of vesicular stomatitis virus is required to shut off cellular RNA synthesis. J Virol. 1979 Apr;30(1):410–413. doi: 10.1128/jvi.30.1.410-413.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Dowling P., Youngner J. S. Alteration of vesicular stomatitis virus L and NS proteins by uv irradiation: implications for the mechanism of host cell shut-off. Virology. 1988 May;164(1):171–175. doi: 10.1016/0042-6822(88)90633-2. [DOI] [PubMed] [Google Scholar]
- Wieben E. D., Nenninger J. M., Pederson T. Ribonucleoprotein organization of eukaryotic RNA. XXXII. U2 small nuclear RNA precursors and their accurate 3' processing in vitro as ribonucleoprotein particles. J Mol Biol. 1985 May 5;183(1):69–78. doi: 10.1016/0022-2836(85)90281-5. [DOI] [PubMed] [Google Scholar]
- Wu F. S., Lucas-Lenard J. M. Inhibition of ribonucleic acid accumulation in mouse L cells infected with vesicular stomatitis virus requires viral ribonucleic acid transcription. Biochemistry. 1980 Feb 19;19(4):804–810. doi: 10.1021/bi00545a029. [DOI] [PubMed] [Google Scholar]
- Youngner J. S., Frielle D. W., Whitaker-Dowling P. Dominance of temperature-sensitive phenotypes. I. Studies of the mechanism of inhibition of the growth of wild-type vesicular stomatitis virus. Virology. 1986 Nov;155(1):225–235. doi: 10.1016/0042-6822(86)90182-0. [DOI] [PubMed] [Google Scholar]
- Yuo C. Y., Ares M., Jr, Weiner A. M. Sequences required for 3' end formation of human U2 small nuclear RNA. Cell. 1985 Aug;42(1):193–202. doi: 10.1016/s0092-8674(85)80115-x. [DOI] [PubMed] [Google Scholar]
- Zieve G. W., Sauterer R. A., Feeney R. J. Newly synthesized small nuclear RNAs appear transiently in the cytoplasm. J Mol Biol. 1988 Jan 20;199(2):259–267. doi: 10.1016/0022-2836(88)90312-9. [DOI] [PubMed] [Google Scholar]