Abstract
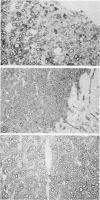
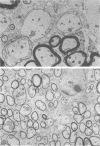
Infection of BALB/c mice with the M variant of encephalomyocarditis virus resulted in the development of a paralytic syndrome in 7 to 10 days. The paralysis was maximal during the period of viral clearance; most of the animals recovered from the initial deficit and showed no delayed recurrences. Pathologically, the white matter of brain and spinal cord showed well-demarcated areas of perivascular cuffing, demyelination, and, during recovery, remyelination by oligodendrocytes--all suggestive of postinfectious encephalomyelitis. Depletion of either the CD4 or CD8 subset of T cells in vivo with the appropriate monoclonal antibody, GK1.5 or 2.43, respectively, administered one day (24 h) prior to infection was sufficient to limit the development of the paralytic syndrome by 79% (GK1.5) and 82% (2.43).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu P. G., Huber S., Sriram S., Craighead J. E. Genetic control of multisystem autoimmune disease in encephalomyocarditis virus infected BALB/cCUM and BALB/cBYJ mice. Curr Top Microbiol Immunol. 1985;122:154–161. doi: 10.1007/978-3-642-70740-7_23. [DOI] [PubMed] [Google Scholar]
- Benjamin R. J., Cobbold S. P., Clark M. R., Waldmann H. Tolerance to rat monoclonal antibodies. Implications for serotherapy. J Exp Med. 1986 Jun 1;163(6):1539–1552. doi: 10.1084/jem.163.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christadoss P., Dauphinee M. J. Immunotherapy for myasthenia gravis: a murine model. J Immunol. 1986 Apr 1;136(7):2437–2440. [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Steinke J. Diabetes mellitus-like syndrome in mice infected with encephalomyocarditis virus. Am J Pathol. 1971 Apr;63(1):119–134. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E. Viral diabetes mellitus in man and experimental animals. Am J Med. 1981 Jan;70(1):127–134. doi: 10.1016/0002-9343(81)90419-8. [DOI] [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G. Experimental models of virus-induced demyelination of the central nervous system. Ann Neurol. 1982 Feb;11(2):109–127. doi: 10.1002/ana.410110202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G. Murine central nervous system infection by a viral temperature-sensitive mutant: a subacute disease leading to demyelination. Am J Pathol. 1981 Mar;102(3):412–426. [PMC free article] [PubMed] [Google Scholar]
- Dixon J. E., Allan J. E., Doherty P. C. The acute inflammatory process in murine lymphocytic choriomeningitis is dependent on Lyt-2+ immune T cells. Cell Immunol. 1987 Jun;107(1):8–14. doi: 10.1016/0008-8749(87)90260-7. [DOI] [PubMed] [Google Scholar]
- Friedmann A., Lorch Y. Theiler's virus infection: a model for multiple sclerosis. Prog Med Virol. 1985;31:43–83. [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. Murine natural killer cells limit coxsackievirus B3 replication. J Immunol. 1987 Aug 1;139(3):913–918. [PubMed] [Google Scholar]
- Huber S. A., Job L. P. Differences in cytolytic T cell response of BALB/c mice infected with myocarditic and non-myocarditic strains of coxsackievirus group B, type 3. Infect Immun. 1983 Mar;39(3):1419–1427. doi: 10.1128/iai.39.3.1419-1427.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Lodge P. A. Coxsackievirus B-3 myocarditis in Balb/c mice. Evidence for autoimmunity to myocyte antigens. Am J Pathol. 1984 Jul;116(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., Griffin D. E., Hirsch R. L., Wolinsky J. S., Roedenbeck S., Lindo de Soriano I., Vaisberg A. Measles encephalomyelitis--clinical and immunologic studies. N Engl J Med. 1984 Jan 19;310(3):137–141. doi: 10.1056/NEJM198401193100301. [DOI] [PubMed] [Google Scholar]
- Lampert P. W. Autoimmune and virus-induced demyelinating diseases. A review. Am J Pathol. 1978 Apr;91(1):176–208. [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge P. A., Herzum M., Olszewski J., Huber S. A. Coxsackievirus B-3 myocarditis. Acute and chronic forms of the disease caused by different immunopathogenic mechanisms. Am J Pathol. 1987 Sep;128(3):455–463. [PMC free article] [PubMed] [Google Scholar]
- Lyden D. C., Huber S. A. Aggravation of coxsackievirus, group B, type 3-induced myocarditis and increase in cellular immunity to myocyte antigens in pregnant Balb/c mice and animals treated with progesterone. Cell Immunol. 1984 Sep;87(2):462–472. doi: 10.1016/0008-8749(84)90015-7. [DOI] [PubMed] [Google Scholar]
- MURNANE T. G., CRAIGHEAD J. E., MONDRAGON H., SHELOKOV A. Fatal disease of swine due to encephalomyocarditis virus. Science. 1960 Feb 19;131(3399):498–499. doi: 10.1126/science.131.3399.498. [DOI] [PubMed] [Google Scholar]
- Matsumori A., Kawai C. Coxsackie virus B3 perimyocarditis in BALB/c mice: experimental model of chronic perimyocarditis in the right ventricle. J Pathol. 1980 Jun;131(2):97–106. doi: 10.1002/path.1711310202. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Jenkins M. K. In vivo effects of GK1.5 (anti-L3T4a) monoclonal antibody on induction and expression of delayed-type hypersensitivity. Cell Immunol. 1985 May;92(2):414–426. doi: 10.1016/0008-8749(85)90022-x. [DOI] [PubMed] [Google Scholar]
- Nakayama E., Uenaka A. Effect of in vivo administration of Lyt antibodies. Lyt phenotype of T cells in lymphoid tissues and blocking of tumor rejection. J Exp Med. 1985 Feb 1;161(2):345–355. doi: 10.1084/jem.161.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Rosenberg A. S., Mizuochi T., Singer A. Analysis of T-cell subsets in rejection of Kb mutant skin allografts differing at class I MHC. 1986 Aug 28-Sep 3Nature. 322(6082):829–831. doi: 10.1038/322829a0. [DOI] [PubMed] [Google Scholar]
- Shanley J. D. Enzyme-linked immunosorbent assay for immunoglobulin G antibody to encephalomyocarditis virus. J Clin Microbiol. 1980 Nov;12(5):663–666. doi: 10.1128/jcm.12.5.663-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S., Carroll L. In vivo depletion of Lyt-2 cells fails to alter acute and relapsing EAE. J Neuroimmunol. 1988 Jan;17(2):147–157. doi: 10.1016/0165-5728(88)90022-7. [DOI] [PubMed] [Google Scholar]
- Sriram S., Roberts C. A. Treatment of established chronic relapsing experimental allergic encephalomyelitis with anti-L3T4 antibodies. J Immunol. 1986 Jun 15;136(12):4464–4469. [PubMed] [Google Scholar]
- Sriram S., Steinman L. Postinfectious and postvaccinial encephalomyelitis. Neurol Clin. 1984 May;2(2):341–353. [PubMed] [Google Scholar]
- Stohlman S. A., Weiner L. P. Chronic central nervous system demyelination in mice after JHM virus infection. Neurology. 1981 Jan;31(1):38–44. doi: 10.1212/wnl.31.1.38. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ceredig R., Cerottini J. C., Louis J. A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically-susceptible BALB/c mice. J Immunol. 1985 Sep;135(3):2108–2114. [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Weiss W. R., Sedegah M., Beaudoin R. L., Miller L. H., Good M. F. CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci U S A. 1988 Jan;85(2):573–576. doi: 10.1073/pnas.85.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh C. J., Tonks P., Nash A. A., Blakemore W. F. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987 Jun;68(Pt 6):1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- Woodruff J. F. Viral myocarditis. A review. Am J Pathol. 1980 Nov;101(2):425–484. [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. I. Polypeptide and ribonucleate components of the virus particle. Virology. 1974 Feb;57(2):531–542. doi: 10.1016/0042-6822(74)90192-5. [DOI] [PubMed] [Google Scholar]