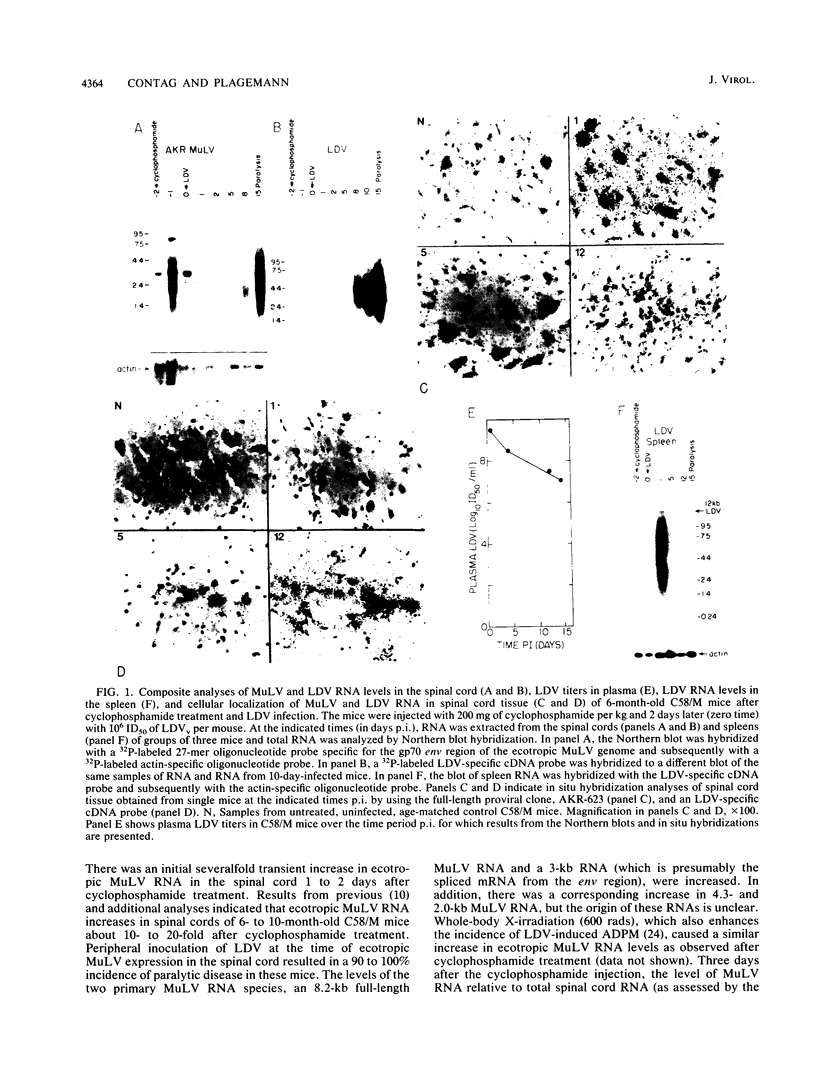
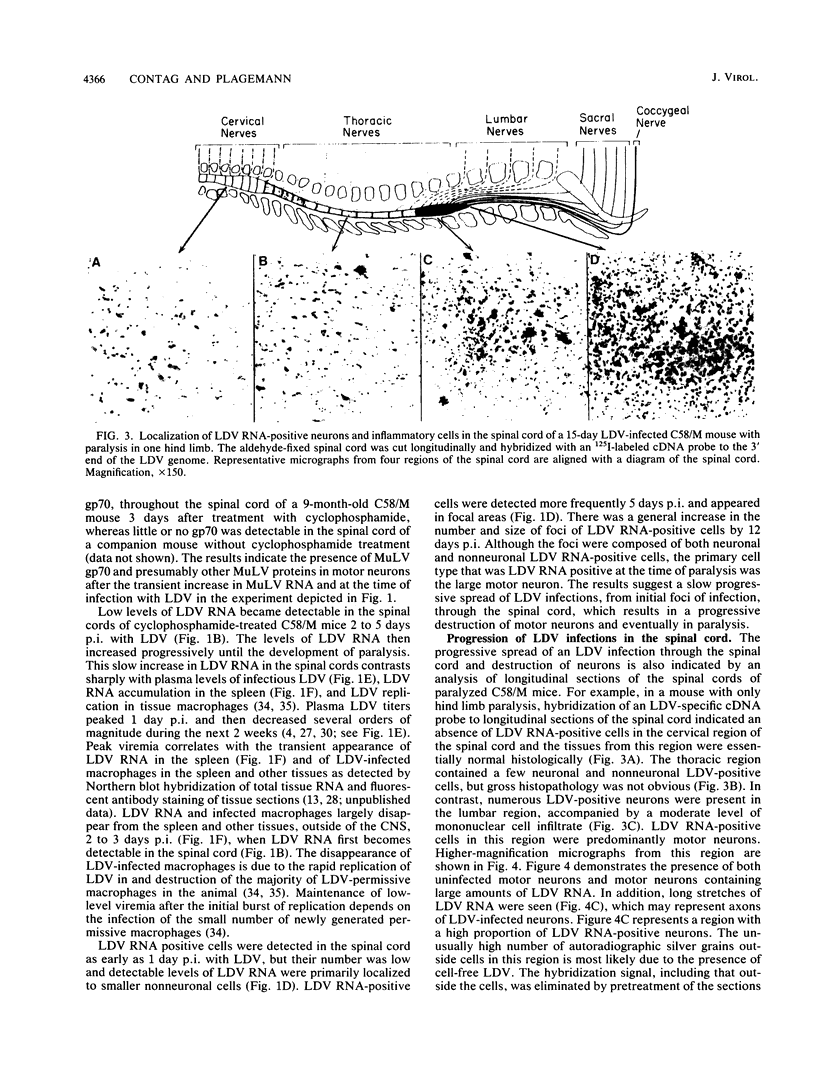
Abstract
The widespread presence of endogenous retroviruses in the genomes of animals and humans has suggested that these viruses may be involved in both normal and abnormal developmental processes. Previous studies have indicated the involvement of endogenous ecotropic murine leukemia virus (MuLV) in the development of age-dependent poliomyelitis caused by infection of old C58 or AKR mice by lactate dehydrogenase-elevating virus (LDV). The only genetic components which segregate with susceptibility to LDV-induced paralytic disease are multiple proviral copies of ecotropic MuLV and the permissive allele, at the Fv-1 locus, for N-tropic, ecotropic virus replication (Fv-1n/n). Using in situ hybridization and Northern (RNA) blot hybridization, we have correlated the expression of the endogenous MuLV, both temporally and spatially, with LDV infection of anterior horn motor neurons and the development of paralysis. Our data indicate that treatment of 6- to 7-month-old C58/M mice with cyclophosphamide, which renders these mice susceptible to LDV-induced paralytic disease, results in transient increases in ecotropic MuLV RNA levels in motor neurons throughout the spinal cord. Peripheral inoculation of C58/M mice with LDV, at the time of elevated MuLV RNA levels, results in a rapid spread of LDV to some spinal cord motor neurons. LDV infections then spread slowly but progressively throughout the spinal cord, involving an increasing number of motor neurons. LDV replication is cytocidal and results in neuron destruction and paralysis of the infected animals 2 to 3 weeks postinfection. The slow replication of LDV in the spinal cord contrasts sharply with the rapid replication of LDV in macrophages, the normal host cells for LDV, during the acute phase of infection. The data indicate that the interaction between the endogenous MuLV with the generally nonpathogenic murine togavirus LDV occurs at the level of the motor neuron. We discuss potential mechanisms for the novel dual-virus etiology of age-dependent poliomyelitis of mice.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum H. E., Haase A. T., Vyas G. N. Molecular pathogenesis of hepatitis B virus infection: simultaneous detection of viral DNA and antigens in paraffin-embedded liver sections. Lancet. 1984 Oct 6;2(8406):771–775. doi: 10.1016/s0140-6736(84)90703-7. [DOI] [PubMed] [Google Scholar]
- Brinton M. A., Gavin E. I., Weibel J. Detection of viral-specific nucleic acid and intracellular virions in ventral horn neurons of lactate dehydrogenase-elevating virus infected C58 mice. Microb Pathog. 1986 Dec;1(6):595–602. doi: 10.1016/0882-4010(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Buxton I. K., Chan S. P., Plagemann P. G. The IA antigen is not the major receptor for lactate dehydrogenase-elevating virus on macrophages from CBA and BALB/c mice. Virus Res. 1988 Feb;9(2-3):205–219. doi: 10.1016/0168-1702(88)90031-7. [DOI] [PubMed] [Google Scholar]
- Cafruny W. A., Strancke C. R., Kowalchyk K., Plagemann P. G. Replication of lactate dehydrogenase-elevating virus in C58 mice and quantification of antiviral antibodies and of tissue virus levels as a function of development of paralytic disease. J Gen Virol. 1986 Jan;67(Pt 1):27–37. doi: 10.1099/0022-1317-67-1-27. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins M. L., Hunsaker W. R. Improved hybridization assays employing tailed oligonucleotide probes: a direct comparison with 5'-end-labeled oligonucleotide probes and nick-translated plasmid probes. Anal Biochem. 1985 Dec;151(2):211–224. doi: 10.1016/0003-2697(85)90168-x. [DOI] [PubMed] [Google Scholar]
- Contag C. H., Chan S. P., Wietgrefe S. W., Plagemann P. G. Correlation between presence of lactate dehydrogenase-elevating virus RNA and antigens in motor neurons and paralysis in infected C58 mice. Virus Res. 1986 Dec;6(3):195–209. doi: 10.1016/0168-1702(86)90069-9. [DOI] [PubMed] [Google Scholar]
- Contag C. H., Plagemann P. G. Susceptibility of C58 mice to paralytic disease induced by lactate dehydrogenase-elevating virus correlates with increased expression of endogenous retrovirus in motor neurons. Microb Pathog. 1988 Oct;5(4):287–296. doi: 10.1016/0882-4010(88)90101-5. [DOI] [PubMed] [Google Scholar]
- Harty J. T., Chan S. P., Contag C. H., Plagemann P. G. Protection of C58 mice from lactate dehydrogenase-elevating virus-induced motor neuron disease by non-neutralizing antiviral antibodies without interference with virus replication. J Neuroimmunol. 1987 Jun;15(2):195–206. doi: 10.1016/0165-5728(87)90093-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann J. W., Steffen D., Gusella J., Tabin C., Bird S., Cowing D., Weinberg R. A. DNA methylation affecting the expression of murine leukemia proviruses. J Virol. 1982 Oct;44(1):144–157. doi: 10.1128/jvi.44.1.144-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T., Mims C. A. Pattern of infection and selective loss of Ia positive cells in suckling and adult mice inoculated with lactic dehydrogenase virus. Arch Virol. 1985;86(3-4):151–165. doi: 10.1007/BF01309821. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., McArthur J. C., Narayan O. The neurobiology of human immunodeficiency virus infections. FASEB J. 1988 Nov;2(14):2970–2981. doi: 10.1096/fasebj.2.14.2846395. [DOI] [PubMed] [Google Scholar]
- Kowalchyk K., Plagemann P. G. Cell surface receptors for lactate dehydrogenase-elevating virus on subpopulation of macrophages. Virus Res. 1985 Apr;2(3):211–229. doi: 10.1016/0168-1702(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Kozak C. A. Retroviruses as chromosomal genes in the mouse. Adv Cancer Res. 1985;44:295–336. doi: 10.1016/s0065-230x(08)60030-5. [DOI] [PubMed] [Google Scholar]
- Laigret F., Repaske R., Boulukos K., Rabson A. B., Khan A. S. Potential progenitor sequences of mink cell focus-forming (MCF) murine leukemia viruses: ecotropic, xenotropic, and MCF-related viral RNAs are detected concurrently in thymus tissues of AKR mice. J Virol. 1988 Feb;62(2):376–386. doi: 10.1128/jvi.62.2.376-386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. E., Sherman T. G., Watson S. J. In situ hybridization histochemistry with synthetic oligonucleotides: strategies and methods. Peptides. 1985;6 (Suppl 2):75–87. doi: 10.1016/0196-9781(85)90138-x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder D. W. Clinical limits of amyotrophic lateral sclerosis. Adv Neurol. 1982;36:15–22. [PubMed] [Google Scholar]
- Murphy W. H., Nawrocki J. F., Pease L. R. Age-dependent paralytic viral infection in C58 mice: possible implications in human neurologic disease. Prog Brain Res. 1983;59:291–303. doi: 10.1016/S0079-6123(08)63874-1. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Riedel N., Hoover E. A., Mullins J. I. Transduction of endogenous envelope genes by feline leukaemia virus in vitro. Nature. 1988 Apr 21;332(6166):731–734. doi: 10.1038/332731a0. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Murphy W. H. Co-infection by lactic dehydrogenase virus and C-type retrovirus elicits neurological disease. Nature. 1980 Jul 24;286(5771):398–400. doi: 10.1038/286398a0. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Porter H. G., Deerhake B. B. Immunofluorescence assay for antigen and antibody in lactic dehydrogenase virus infection of mice. J Immunol. 1969 Feb;102(2):431–436. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ritzi D. M., Holth M., Smith M. S., Swart W. J., Cafruny W. A., Plagemann G. W., Stueckemann J. A. Replication of lactate dehydrogenase-elevating virus in macrophages. 1. Evidence for cytocidal replication. J Gen Virol. 1982 Apr;59(Pt 2):245–262. doi: 10.1099/0022-1317-59-2-245. [DOI] [PubMed] [Google Scholar]
- Rowson K. E., Mahy B. W. Lactate dehydrogenase-elevating virus. J Gen Virol. 1985 Nov;66(Pt 11):2297–2312. doi: 10.1099/0022-1317-66-11-2297. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. The four classes of endogenous murine leukemia virus: structural relationships and potential for recombination. J Virol. 1987 Sep;61(9):2659–2669. doi: 10.1128/jvi.61.9.2659-2669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Fenner S., Greenoak G. E., Moran C., Coffin J. M. Role of endogenous retroviruses as mutagens: the hairless mutation of mice. Cell. 1988 Jul 29;54(3):383–391. doi: 10.1016/0092-8674(88)90201-2. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Weibel J., Schaefer D., Brinton M. A. Ultrastructural and immunofluorescent studies of acute and chronic lactate dehydrogenase elevating virus-induced nonparalytic poliomyelitis in mice. Proc Soc Exp Biol Med. 1985 Feb;178(2):261–274. doi: 10.3181/00379727-178-42009. [DOI] [PubMed] [Google Scholar]
- Stueckemann J. A., Holth M., Swart W. J., Kowalchyk K., Smith M. S., Wolstenholme A. J., Cafruny W. A., Plagemann P. G. Replication of lactate dehydrogenase-elevating virus in macrophages. 2. Mechanism of persistent infection in mice and cell culture. J Gen Virol. 1982 Apr;59(Pt 2):263–272. doi: 10.1099/0022-1317-59-2-263. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., McPhee D. A., Fields B. N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986 Aug 15;233(4765):770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Varmus H. Retroviruses. Science. 1988 Jun 10;240(4858):1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]