Abstract
In the South West Pacific region, the striking geographical correlation between the frequency of α+-thalassemia and the endemicity of Plasmodium falciparum suggests that this hemoglobinopathy provides a selective advantage against malaria. In Vanuatu, paradoxically, α+-thalassemia increases the incidence of contracting mild malaria in the first 2 years of life, but severe disease was too uncommon to assess adequately. Therefore, we undertook a prospective case-control study of children with severe malaria on the north coast of Papua New Guinea, where malaria transmission is intense and α+-thalassemia affects more than 90% of the population. Compared with normal children, the risk of having severe malaria was 0.40 (95% confidence interval 0.22–0.74) in α+-thalassemia homozygotes and 0.66 (0.37–1.20) in heterozygotes. Unexpectedly, the risk of hospital admission with infections other than malaria also was reduced to a similar degree in homozygous (0.36; 95% confidence interval 0.22–0.60) and heterozygous (0.63; 0.38–1.07) children. This clinical study demonstrates that a malaria resistance gene protects against disease caused by infections other than malaria. The mechanism of the remarkable protective effect of α+-thalassemia against severe childhood disease remains unclear but must encompass the clear interaction between this hemoglobinopathy and both malarial and nonmalarial infections.
The inherited disorders of hemoglobin, especially thalassemia and some structural variants like HbS and HbE, are the most common monogenic disorders of humans (1). The thalassemias are a heterogeneous group of disorders that result from the reduced or absent synthesis of the α- or β-globin chains that constitute normal adult hemoglobin (α2β2). There are two α-globin genes on each chromosome 16 (αα/αα) and, in α+-thalassemia, one gene of the pair is deleted (−α). The clinical effects are modest; reduced α-globin chain synthesis in homozygotes (−α/−α) causes only mild anemia (average hemoglobin levels 1–2 g/dl lower than normals), and the hemoglobin level and red cell indices in heterozygotes (−α/αα) are often indistinguishable from normal (1). There are less common, “nondeletional” forms of α+-thalassemia caused by mutations that reduce the output of one or the other α-globin genes. Both variants are associated with increased levels of the γ4 tetramer Hb Bart’s in the neonatal period, a reflection of excess production of the γ chains of fetal hemoglobin (α2γ2).
The suggestion that α+-thalassemia has been selected by malaria is based mainly on epidemiological studies. There is a close altitude- and latitude-dependent correlation between the frequency of α+-thalassemia and the endemicity of Plasmodium falciparum in the South West Pacific (2, 3). Similarly, a marked decreased incidence of malaria in the Tharu people of southern Nepal compared with synpatric non-Tharu, based on a retrospective analysis of records from a malaria control program (4), and their ability to inhabit a malaria-holoendemic region, was ascribed to a high incidence of α+-thalassemia (5). A small prospective cohort study of Gambian children showed no evidence that α+-thalassemia heterozygotes were protected from either asymptomatic parasitemia or clinical episodes of malaria (6).
We investigated the relationship between α+-thalassemia and childhood illnesses in Madang Province, the north coastal region of Papua New Guinea. Here, α+-thalassemia is very common (homozygotes comprise approximately 55% and heterozygotes 37% of the local population; refs. 2, 3, and 7), and P. falciparum is hyperendemic and transmitted throughout the year (8). A previous village-based study of Madang children aged less than 10 years estimated that 11% of deaths were attributable to malaria (9). The primary hypothesis that α+-thalassemia protects against death from malaria was tested in a prospective, matched case-control study. Because death caused by malaria is uncommon, we used admission to a hospital with severe malaria as a surrogate for malaria mortality. To investigate the effect against other illnesses, the frequency of α-globin gene deletions also was determined in children with mild malaria and mild and severe nonmalarial diseases.
METHODS
The study was undertaken between October 1993 and February 1996 and was based on the pediatric ward of the Madang hospital. Most of the local population live in rural villages while migrants from other regions of Papua New Guinea live in Madang town or peri-urban settlements. Only individuals who had lived in Madang Province for at least 1 year were included. Residence was coded according to the geographical regions described in the Papua New Guinea 1990 National Population Census (National Statistics Office, Port Moresby; Fig. 1) and ethnicity according to the local languages spoken by an individual’s parents. Malaria control measures consist only of the presumptive treatment of fever.
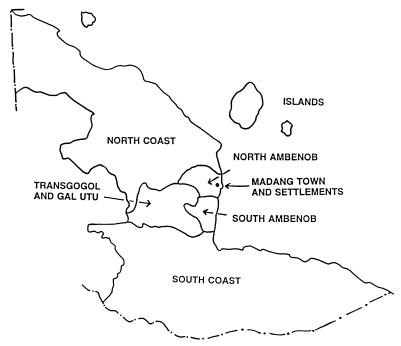
Figure 1.
Diagram of study area. Fewer children were recruited from regions distant from the hospital and, therefore, these children have been amalgamated into “North Coast,” “South Coast,” and “Islands.” The total population of each region according to the 1990 National Population Census and gene frequency for α− (± standard error) based on all children recruited in this study were as follows: Islands, 5,157 (0.50 ± 0.077); Madang Town and settlements, 4,346 (0.59 ± 0.019); North Ambenob, 1,477 (0.63 ± 0.026); North Coast, 10,665 (0.60 ± 0.028); South Ambenob, 2,285 (0.73 ± 0.021); South Coast, 17,859 (0.52 ± 0.039), and Transgogol and Gal Utu, 10,515 (0.64 ± 0.010).
Case-Control Study.
Protection against severe malaria, a relatively uncommon disease, was assessed by using an individually matched case-control study design (10). Index cases were children admitted to Madang hospital with one or more severe manifestations of malaria defined according to World Health Organization criteria (11, 12). Briefly, coma was defined as a Blantyre coma score of ≤2 with asexual stages of P. falciparum present in peripheral blood and no evidence of bacterial or viral meningoencephalitis, and acidosis was defined as plasma bicarbonate <15 mmol/liter, hypoglycemia as plasma glucose <2.2 mmol/liter and hyperlactatemia as plasma lactate ≥5 mmol/liter. Clinical experience revealed that causes of severe anemia (hemoglobin <5 g/dl) other than malaria were uncommon. Intestinal infection with Necator americanus does not cause reduced Hb levels in this population because worm burdens are low (13). Nearly all children with severe anemia presented during an acute febrile illness, and most had taken amodiaquine before admission (12), which would be expected to have reduced but not cleared parasitemia (14). Therefore, the parasitological criteria in the World Health Organization case definition for severe malarial anemia (asexual P. falciparum parasitemia ≥10,000/μl; ref. 11) was modified to include children with a lower density of falciparum parasitemia if they had taken amodiaquine before admission. The decision to admit children was made by local health workers who were not employed by the project. The methods used to recruit children on admission to the ward, and the clinical features in those with severe malaria, have been published previously (12).
One matched community control child was recruited for each index case. A trained field worker randomly selected the house of an unrelated family neighboring that of the index case by spinning a pencil. A child of the same sex, age difference <1 year and with both parents of the same ethnicity as the index case from this house, or from the next house in a clockwise direction if no suitable child was available, was recruited (10).
Other Clinical Groups.
Indices of the severity of malarial infection and mortality were compared according to genotype for α+-thalassemia in all children admitted to the hospital with malaria. Children admitted during the routine clinical duties of the research physician (S.J.A.) with a clinical diagnosis of an illnesses other than malaria and without malaria parasitemia were recruited as “severe nonmalaria cases” and the clinical diagnosis recorded. Children with mild illness were recruited by a field worker who visited fortnightly each of six clinics situated in the hospital catchment area. Blood sugar was measured to exclude hypoglycemia (BM test 1–44; Boehringer Mannheim) and no child was considered to require hospital admission by the local health workers. “Mild malaria” cases were those with a febrile illness and P. falciparum parasitemia ≥10,000/μl, and “mild nonmalaria cases” were those with a febrile illness but negative blood films. Genotype for α+-thalassemia also was determined in local adults of Madang ethnicity (recruited from blood donor clinics and volunteers encountered during the field worker’s visits to villages). The level of Hb Bart’s in cord blood collected from infants delivered in Madang hospital was compared with their α-globin genotype to assess the frequency of nondeletional α+-thalassemia.
Laboratory Methods.
Methods of blood collection and hematological and biochemical analyses have been published previously (12). Analysis for glucose-6-phosphate dehydrogenase deficiency (Sigma, procedure no. 400), measurement of hemoglobin A2 (Helena Kwik-Column) and hemoglobin electrophoresis were performed on all samples. Genotype for α+-thalassemia was determined by Southern blotting (15) and the band III deletion for Southeast Asian ovalocytosis by PCR (16). All clinical diagnoses and recruitment of children were completed before the genotypes were known.
Statistical Analysis.
Conditional logistic regression analysis, with case/control status as the outcome variable, was performed on the index case-community control pairs in which the genotype for α+-thalassemia had been determined in both members of the pair (10). This iterative maximum-likelihood technique estimates simultaneously the effects of multiple potential risk factors for severe malaria. In particular, it provides joint odds ratios for both α+-thalassemia homozygotes and heterozygotes relative to normals, with age and ethnicity also in the model as potential confounding factors. For the other clinical groups, lack of individual matching meant that the logistic regression was not conditional, but otherwise the effect of α+-thalassemia was assessed similarly. Each group was compared with the community controls, with age, ethnicity and residence included as possible confounders. egret (SERC, Seattle, WA) was used for all regression analysis. All analyses were repeated, including presence of deletion of the band III gene for Southeast Asian ovalocytosis to check for confounding from this red cell abnormality, and unadjusted odds ratios also were calculated from the crude case-control data (17) to check for invalid assumptions in regression models. In all children admitted to the hospital with malaria, categorical clinical variables according to genotype for α+-thalassemia were compared by χ2 test for trend, and continuous laboratory variables were compared by the Kruskal–Wallis test.
RESULTS
As expected, the frequency of −α varied markedly with ethnicity [frequency in cord blood samples (n) where both parents were of the same ethnicity: Madang 0.68 (225 cases), Sepik 0.87 (58 cases), other coastal areas 0.35 (26 cases), and Highlands 0.0 (13 cases)]. Hb Bart’s in cord blood was <2.8% in 38 −α/αα infants and was detected in one of 25 αα/αα infants who had a value of 1.8%. These results correspond to a gene frequency for nondeletional α+-thalassemia of 1.1%. The band III deletion for Southeast Asian ovalocytosis was detected in 6.9% of all children and was not associated with genotype for α+-thalassemia (χ2 = 3.53, 2 degrees of freedom, P = 0.17). One mild malaria case and three community controls had β-thalassemia trait (HbA2 > 3.1%). Hb J Tongariki was detected in one severe malaria, two severe nonmalaria, two mild nonmalaria cases, and three community control children. Among boys, deficiency of glucose-6-phosphate dehydrogenase was detected in three of 161 (1.9%) severe malaria cases, one of 59 (1.7%) mild malaria, five of 107 (4.7%) severe nonmalaria, zero of 65 mild nonmalaria, and four of 170 (2.4%) community controls.
Case-Control Study.
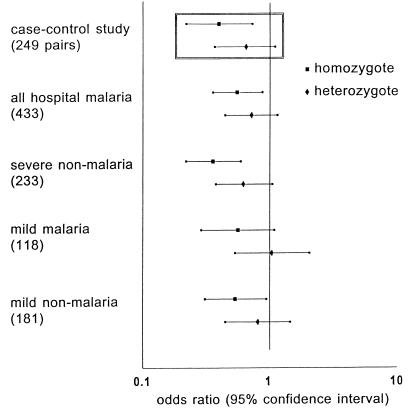
The genotype for α+-thalassemia was determined successfully in 433 of 489 children admitted to the hospital with malaria and in 289 community controls and, thereby, in both members of 249 index case-community control pairs. Conditional logistic regression analysis showed that, compared with normal children, the risk of severe malaria was 0.40 in α+-thalassemia homozygotes and 0.66 in heterozygotes (Table 1 and Fig. 2). Subgroup analysis suggested that homozygous α+-thalassemia protected against all manifestations of severe malaria except for coma and hypoglycemia with the lowest odds ratios observed in the acidosis and hyperlactatemia groups (Table 1). Protection against severe anemia also was observed when the more strict World Health Organization criteria for severe malaria anemia (11) was used (Hb < 5 g/dl and P. falciparum parasitemia >10,000/μl; 84 case-control pairs; odds ratio [95% confidence interval] for homozygotes: 0.25 [0.080–0.77]; heterozygotes: 0.80 [0.30–2.14]). Neither calculation of unadjusted odds ratios from the crude case-control data (17), nor inclusion of deletion of the band III gene for Southeast Asian ovalocytosis in the regression model, materially altered the results. A similar degree of clinical protection was observed when all children admitted to the hospital with malaria were compared with the community controls in logistic regression analysis (Fig. 2).
Table 1.
Conditional logistic regression analysis of matched severe malaria–community control pairs
| Groups | Number of pairs
|
|||||
|---|---|---|---|---|---|---|
| Cases | Controls
|
Odds ratio (95% CI) [P value]
|
||||
| −α/−α | −α/αα | αα/αα | −α/−α | −α/αα | ||
| Severe malaria group (249 pairs) | −α/−α | 72 | 35 | 4 | 0.40 (0.22–0.74) | 0.66 (0.37–1.2) |
| −α/αα | 48 | 28 | 17 | [0.003] | [0.2] | |
| αα/αα | 22 | 15 | 8 | |||
| Clinical subgroups | ||||||
| Severe anemia (155) | −α/−α | 37 | 16 | 3 | 0.34 (0.16–0.73) | 0.84 (0.41–1.7) |
| −α/αα | 36 | 20 | 12 | [0.004] | [0.6] | |
| αα/αα | 15 | 10 | 6 | |||
| Coma (56) | −α/−α | 19 | 10 | 0 | 0.90 (0.19–4.3) | 1.27 (0.31–5.2) |
| −α/αα | 9 | 6 | 5 | [0.90] | [0.7] | |
| αα/αα | 2 | 4 | 1 | |||
| Hypoglycemia (13) | −α/−α | 2 | 2 | 0 | 1.75 (0.023–135) | 4.90 (0.078–307) |
| −α/αα | 5 | 2 | 1 | [0.8] | [0.4] | |
| αα/αα | 1 | 0 | 0 | |||
| Acidosis (42) | −α/−α | 19 | 4 | 0 | 0 (0–0.028) | 0 (0–0.14) |
| −α/αα | 9 | 3 | 0 | [0.0006] | [0.005] | |
| αα/αα | 3 | 2 | 2 | |||
| Hyperlactatemia (62) | −α/−α | 22 | 9 | 1 | 0.25 (0.057–1.1) | 0.32 (0.070–1.4) |
| −α/αα | 9 | 6 | 4 | [0.06] | [0.1] | |
| αα/αα | 5 | 4 | 2 | |||
Conditional logistic regression analysis was performed with case-control status as the outcome variable and age and ethnicity included in the regression model. The middle columns show case-control pairs according to genotype for α+-thalassemia. In the middle columns, only pairs discordant in genotype (shown in bold type) contribute to the analysis. An approximate assessment of the degree of protection afforded by α+-thalassemia can be obtained from these raw data. For example, considering the 26 homozygote/normal pairs in the severe malaria group, the homozygous member of the pair was the control in 22 and the case in only four pairs whereas an approximately equal distribution would be expected if homozygous α+-thalassemia conferred no protection against severe malaria. However, more accurate estimates are obtained by using all the discordant pairs in a single analysis, because the three comparisons of homozygote vs. normal, heterozygote vs. normal, and homozygote vs. heterozygote are mutually dependent. The odds ratios shown represent the risk of developing severe malaria for homozygotes and heterozygotes relative to normals. To investigate the protective effect of α+-thalassemia against individual manifestations of severe malaria, conditional logistic regression analysis was repeated selecting only the pairs in which the case had a particular severe manifestation of malaria. Median (range) age was 2.1 (0.2–12.4) years for children with severe anemia, 3.5 (0.8–11.7) for coma, 3.0 (0.5–7.0) for hypoglycemia, 2.4 (0.5–9.5) for acidosis, and 2.6 (0.2–9.9) for hyperlactatemia. Many children had more than one severe manifestation of malaria (12). A sole severe manifestation of malaria was detected in 145 of 179 children in whom it was possible to assess all severe manifestations (103 with severe anemia, 24 with coma, 3 with hypoglycemia, 14 with acidosis, and 1 with hyperlactatemia).
Figure 2.
Risk of clinical disease compared with community controls according to genotype for α+-thalassemia. Odds ratios with 95% confidence intervals represent the risk for children with α+-thalassemia of developing each of the clinical outcomes relative to normals. Numbers in each group are shown in parentheses. (Inset) Results from the matched index case-community control analysis (Table 1).
Two hundred and thirty one (93%) of the controls were recruited within 1 month of admission of the index case. Community controls should be representative of the source population from which the index cases are drawn (10). The method used to recruit controls appeared to have achieved this representation in respect to the frequency of −α in that, in individuals of Madang ethnicity, the −α frequency in the community controls [0.71 (standard error 0.023)] was similar to that in 215 adult blood donors and volunteers [0.73 (0.022)]). Average hemoglobin levels and malariometric indices according to genotype for α+-thalassemia in the community control children are shown in Table 2. Apart from more frequent P. vivax infection among homozygous children, no significant differences were observed.
Table 2.
Hemoglobin and malariometric indices according to genotype for α+-thalassemia in community control children
| Indices | −α/−α | −α/αα | αα/αα | P value |
|---|---|---|---|---|
| Hemoglobin, g/dl | 9.9 (9.0–10.8) | 10.3 (9.0–11.2) | 10.4 (8.6–11.5) | 0.15 |
| [156] | [87] | [37] | ||
| P. falciparum rate | 63/160 (39.4) | 36/89 (40.5) | 15/36 (41.7) | 0.96 |
| Density, log10 value/μl | 3.1 (2.4–3.8) | 3.3 (2.5–3.9) | 3.2 (2.3–3.7) | 0.82 |
| P. vivax rate | 35/160 (21.9) | 6/89 (6.7) | 4/36 (11.1) | 0.0052 |
| Density, log10 value/μl | 2.5 (2.2–3.2) | 2.8 (2.5–3.5) | 2.1 (2.0–2.9) | 0.43 |
| Spleen (Hackett grade) | ||||
| 0 | 92 (56.8) | 48 (53.3) | 18 (48.7) | |
| 1–3 | 68 (42.0) | 37 (41.1) | 15 (40.5) | 0.068 |
| 4–5 | 2 (1.2) | 5 (5.6) | 4 (10.8) |
Continuous variables are shown as median value (inter-quartile range) and categorical variables as number (%). χ2 test for trend for parasite rates and splenomegaly and Kruskal–Wallis test for other variables.
Severe Nonmalaria Cases.
Two hundred and thirty three severe nonmalaria cases were recruited, and they differed from the community controls in respect to median age (P < 0.001) and ethnicity (P = 0.040) although region of residence was similar (P = 0.090; Table 3). Surprisingly, in logistic regression analysis accounting for these differences, the risk of being a severe nonmalaria case rather than a community control was 0.36 (95% confidence interval 0.22–0.60; P < 0.001) for homozygotes and 0.63 (0.38–1.07; P = 0.084) for heterozygotes (Fig. 2). Most of these severe nonmalaria cases were admitted with acute infectious illnesses. The most frequent diagnoses were respiratory infection (n = 91), gastroenteritis (n = 22), and meningitis (n = 20) and, in children of Madang ethnicity, the −α frequency (SE) in each of these groups [0.61 (0.046), 0.58 (0.10), and 0.64 (0.080), respectively] was similar to that in all severe nonmalaria cases of Madang ethnicity [n = 142; 0.58 (0.029)].
Table 3.
Demographic characteristics and genotype for α+-thalassemia according to clinical group
| All hospital malaria n = 433 | Mild malaria n = 118 | Severe nonmalaria n = 233 | Mild nonmalaria n = 181 | Community controls n = 289 | |
|---|---|---|---|---|---|
| Age (years) | 2.8 (1.7–4.3) | 3.6 (2.2–5.9) | 1.6 (0.8–4.5) | 3.3 (1.5–6.1) | 3.0 (1.7–4.5) |
| Sex | 229 (52.9) | 67 (56.8) | 127 (54.5) | 100 (55.3) | 156 (54.0) |
| Ethnicity | |||||
| Madang | 22 (63.0) | 72 (61.5) | 142 (61.7) | 106 (58.9) | 205 (70.9) |
| Sepik | 56 (13.0) | 14 (12.0) | 21 (9.1) | 21 (11.7) | 30 (10.4) |
| Other coastal | 10 (2.3) | 3 (2.6) | 7 (3.0) | 9 (5.0) | 5 (1.7) |
| Highlands | 11 (2.6) | 2 (1.7) | 9 (3.9) | 2 (1.1) | 3 (1.0) |
| Mixed | 83 (19.2) | 26 (22.2) | 51 (22.2) | 42 (23.3) | 46 (15.9) |
| Residence | |||||
| Madang town or settlement | 140 (32.4) | 37 (31.4) | 81 (34.9) | 59 (32.6) | 81 (28.0) |
| North Ambenob | 67 (15.5) | 19 (16.1) | 30 (12.9) | 40 (22.1) | 43 (14.9) |
| South Ambenob | 87 (20.1) | 26 (22.0) | 39 (16.8) | 41 (22.7) | 57 (19.7) |
| Transgogol and Gal Utu | 44 (10.2) | 6 (5.1) | 24 (10.4) | 6 (3.3) | 39 (13.5) |
| North coast | 50 (11.6) | 24 (20.3) | 25 (10.8) | 27 (14.9) | 43 (14.9) |
| South coast | 38 (8.8) | 5 (4.2) | 24 (10.4) | 4 (2.2) | 23 (8.0) |
| Islands | 6 (1.4) | 1 (0.9) | 9 (3.9) | 4 (2.2) | 3 (1.0) |
| α+-Thalassemia genotype | |||||
| −α/−α | 202 (46.7) | 46 (39.0) | 89 (38.2) | 77 (42.5) | 162 (56.1) |
| −α/αα | 146 (33.7) | 53 (44.9) | 85 (36.5) | 69 (38.1) | 90 (31.1) |
| αα/αα | 85 (19.6) | 19 (16.1) | 59 (25.3) | 35 (19.3) | 37 (12.8) |
See Methods section for definitions of clinical groups. Median (inter-quartile range) is shown for age; other data are number (%). Mixed ethnicity was when the child’s mother was from a different ethnic group than the child’s father. Ethnicity was not known for one hospital malaria, one mild malaria, one mild nonmalaria, and three severe nonmalaria cases. The exact residence was not known for one hospital malaria and one nonmalaria severe case.
Mild Cases.
One hundred and eighteen children with mild malaria and 181 children attending the clinic with fever but negative blood films (mild nonmalaria cases) were recruited. Compared with community controls, the risk for homozygous children of being a mild nonmalaria case was 0.54 (95% confidence interval 0.31–0.96, P = 0.036) and of being a mild malaria case was 0.57 (0.29–1.11, P = 0.10; Fig. 2).
All Children Admitted to the Hospital with Malaria.
Table 4 shows hemoglobin, biochemical indices, parasitemia, and mortality in 433 children admitted to the hospital with malaria (including index cases). There were no statistically significant differences in any of these parameters according to genotype for α+-thalassemia.
Table 4.
Indices of severity of malaria and mortality according to genotype for α+-thalassemia in 433 children admitted to the hospital with malaria
| −α/−α | αα/−α | αα/αα | P value | |
|---|---|---|---|---|
| Hb, g/dl | 6.5 (1.4–13.4) | 5.2 (2.3–16.6) | 5.8 (2.4–13.9) | 0.097 |
| [202] | [146] | [85] | ||
| Glucose, mmol/liter | 4.9 (1.0–10.1) | 4.8 (1.0–12.4) | 5.1 (2.1–14.6) | 0.54 |
| [193] | [135] | [84] | ||
| Bicarbonate, mmol/liter | 20 (8–33) | 21 (9–35) | 21 (7–31) | 0.57 |
| [191] | [131] | [83] | ||
| Lactate, mmol/liter | 3.1 (1.2–15.6) | 2.9 (1.4–11.1) | 2.7 (1.4–8.9) | 0.53 |
| [188] | [135] | [80] | ||
| P. falciparum, log10 value/μl | 4.4 (1.4–5.9) | 4.3 (1.5–5.8) | 4.1 (1.8–5.7) | 0.33 |
| [196] | [140] | [81] | ||
| Coma | 32/197 (16.2) | 24/144 (16.7) | 7/85 (8.2) | 0.16 |
| Deaths | 9/202 (4.5) | 6/146 (4.1) | 0/85 (0) | 0.15 |
For coma and deaths, percentages are shown in brackets and analysis is χ2 test for trend. For other variables, median values with inter-quartile range in brackets and number of observations in square brackets are shown, and analysis is by Kruskal–Wallis test.
DISCUSSION
α+-Thalassemia is extremely common in the north coastal region of Papua New Guinea (2, 3, 7), and we have demonstrated that it protects against severe malaria. Although severe malaria was used as a surrogate for direct malaria mortality, the lowest odds ratios for α+-thalassemia homozygotes were present in the acidosis and hyperlactatemia sub-groups. We have shown previously that these complications of malaria were the most strongly predictive of mortality (12). Furthermore, the occurrence of nondeletional α+-thalassemia in this population means that this study will have underestimated the true protective effect of this hemoglobinopathy against clinical disease. Although not assessed in this study, the protection against complications that were most strongly associated with mortality also suggests that α+-thalassemia is likely to prevent rapidly fulminating disease, which may cause mortality before children reach the hospital. The protection against malaria demonstrated in Papua New Guinean children is entirely consistent with the marked reduced morbidity from malaria ascribed to α-globin gene deletions in the Tharu people of southern Nepal (5).
Glucose-6-phosphate dehydrogenase deficiency (18) and β-thalassemia trait (19, 20) also are associated with protection against malaria but were uncommon in this population. Interestingly, α+-thalassemia did not appear to prevent coma; however, we have reported previously that, rather than having a single pathogenesis, coma may result from several complications of severe malaria (21), and this finding may account for the apparent lack of protection. In contrast, the band III deletion for Southeast Asian ovalocytosis has been associated with protection against cerebral malaria in Madang children (22). This deletion did not contribute to the protection observed by α+-thalassemia because the two abnormalities were not associated and including deletion of the band III gene in the regression analysis did not alter the results.
Several in vitro studies have investigated the mechanism of resistance to malaria in α+-thalassemia. P. falciparum has been shown to thrive well in thalassaemic red cells (23) although a recent study has shown a decreased development during later parasite cycles, with several factors possibly contributing to schizont maturation arrest (24). The findings in this study of a similar frequency and density of P. falciparum in the community controls according to genotype agree with these in vitro studies and suggest that α+-thalassemia does not impair the development of blood-stage infection. Certainly, comparison of clinical features and biochemical indices in children admitted to hospital with malaria indicated that α+-thalassemia did not appear to alter the course of disease once it had developed to a sufficient degree to require hospital admission.
The mechanism whereby α+-thalassemia protects against malaria may have an immunological basis. Parasitised α+-thalassaemic erythrocytes bound greater levels of antibody from malaria endemic sera (25) and were more readily phagocytosed by blood monocytes (26) compared with control cells. A greater frequency of malaria in young children with thalassemia than normals has been found both in Papua New Guinea (27) and Vanuatu (28). In the latter study, P. vivax was increased particularly in children aged <30 months, and it was proposed that this increase may act as a natural vaccine against P. falciparum (28). These findings appear to be confirmed by the present study in that P. vivax infection in the community control children was more common in homozygous α+-thalassemia. The increased reticulocytosis in α+-thalassemia (1) may underlie this increased susceptibility to P. vivax infection because this parasite only infects reticulocytes. The age at which clinical immunity develops, as a consequence of exposure to infection, would be expected to be determined by the intensity of transmission of malaria in the locality. In this study, a tendency toward reduced mild malaria in homozygous α+-thalassemia was observed in children with a median age of 3.6 years and reduced admission to the hospital with malaria in children with a median age of 2.8 years. This finding suggests that, in this malaria hyperendemic region, the period of increased susceptibility to malaria that may precede clinical protection in α+-thalassemia may be quite short-lived.
The reduction in nonmalaria illnesses in children with α+-thalassemia was unexpected but striking. Although this study was not designed to investigate protection against illnesses other than malaria, the children recruited in the community appeared to be a satisfactory control group for the severe nonmalaria cases. The regions of residence of the two groups were similar, suggesting that access to the hospital was similar, and differences in age and ethnicity were accounted for in the analysis. This study demonstrates protection by a malaria resistance gene against illnesses other than malaria. Although the mechanisms discussed above may account for the prevention of malaria in α+-thalassemia, how protection against disease because of nonmalarial infections may occur is unclear.
A reduction in mortality greater than that attributable directly to malaria has been observed after the prevention of malaria by insecticides (29), chemoprophylaxis (30), and insecticide-impregnated bed nets (reviewed in ref. 31). Similarly, α+-thalassemia may have reduced disease caused by nonmalaria infections as a result of the prevention of acute malaria and the accompanying immunosuppression (32) or from the prevention of severe malaria anemia. The latter seems unlikely in that α+-thalassemia causes mild anemia and hemoglobin levels according to genotype did not differ in the community control children.
A further, nonexclusive, possibility is that the acquired anti-disease immunity to malaria in α+-thalassemia may have a nonspecific immunological component, which also reduces disease caused by some other infections. Pro-inflammatory cytokines, such as tumor necrosis factor, are central disease mediators both in malaria (reviewed in ref. 33) and the systemic inflammatory response syndrome (34). Abrogation of the pathogenic effects of high levels of cytokines may be a component of acquired anti-disease immunity in malaria and also may reduce disease caused by other infections. Some support for this hypothesis comes from studies in human volunteers where exposure to malaria reduced the febrile response to subsequent endotoxin challenge (35). Alternatively, exposure to some infections early in life has been associated with enhanced cellular immune responses in older children (36, 37), and the apparent increased frequency of P. vivax in α+-thalassemia may modulate immune responses to afford greater protection against some common pathogens.
Although it is appropriate to study severe cases of malaria to detect genetic influences (38), the findings in this study highlight the importance of considering total malaria mortality, the sum of direct and indirect malaria mortality, when assessing the survival advantage conferred by α+-thalassemia: indirect malaria mortality may be equal to or even greater than direct malaria mortality (39). Previous observations that direct malaria mortality cannot account for observed HbS gene frequencies (40), suggest that this finding may apply equally to other malaria resistance genes. Given its remarkably high frequency and the combined protection it affords against illness caused by malaria and some other infections, α+-thalassemia is likely to have a major impact on child survival in coastal Papua New Guinea.
Acknowledgments
We are indebted to the people of Madang who took part in our studies. We thank W. Deppsone for meticulous field work, M. Mellombo, A. Raiko, A. Mai, M. J. Rugless, and N. H. Chapman for laboratory assistance, Professor D. A. Warrell for advice and help in setting up this project, and T. N. Williams and K. Maitland for comments on the manuscript. This work was supported by the Wellcome Trust and the Medical Research Council.
References
- 1.Weatherall D J, Clegg J B. The Thalassaemia Syndromes. Oxford: Blackwell; 1981. [Google Scholar]
- 2.Flint J, Hill A V S, Bowden D K, Oppenheimer S J, Sill P R, Serjeantson S W, Bana-Koiri J, Bhatia K, Alpers M P, Boyce A J, Weatherall D J, Clegg J B. Nature (London) 1986;321:744–750. doi: 10.1038/321744a0. [DOI] [PubMed] [Google Scholar]
- 3.Yenchitsomanus P, Summers K M, Board P G, Bhatia K, Jones G L, Johnston K, Nurse G T. Hum Genet. 1986;74:432–437. doi: 10.1007/BF00280500. [DOI] [PubMed] [Google Scholar]
- 4.Terrenato L, Shrestha S, Dixit K A, Luzzatto L, Modiano G, Morpurgo G, Arese P. Ann Trop Med Parasitol. 1988;82:1–11. doi: 10.1080/00034983.1988.11812202. [DOI] [PubMed] [Google Scholar]
- 5.Modiano G, Morpurgo G, Terrenato L, Novelletto A, Di Rienzo A, Colombo B, Purpura M, Mariani M, Santachiara-Benerecetti S, Brega A, Dixit K A, Shrestha S L, Lania A, Wanachiwanawin W, Luzzatto L. Am J Hum Genet. 1991;48:390–397. [PMC free article] [PubMed] [Google Scholar]
- 6.Allen S J, Rowe P, Allsopp C E M, Riley E M, Jakobsen P H, Hill A V S, Greenwood B M. Trans R Soc Trop Med Hyg. 1993;87:282–285. doi: 10.1016/0035-9203(93)90129-e. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer S J, Higgs D R, Weatherall D J, Barker J, Spark R A. Lancet. 1984;i:424–426. doi: 10.1016/s0140-6736(84)91754-9. [DOI] [PubMed] [Google Scholar]
- 8.Cattani J A, Tulloch J L, Vrbova H, Jolley D, Gibson F D, Moir J S, Heywood P F, Alpers M P, Stevenson A, Clancy R. Am J Trop Med Hyg. 1986;35:3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- 9.Moir J S, Tulloch J L, Vrbova H, Jolley D J, Heywood P F, Alpers M P. PNG Med J. 1985;28:267–278. [PubMed] [Google Scholar]
- 10.Hayes R J, Marsh K, Snow R W. J Trop Med Hyg. 1992;95:157–166. [PubMed] [Google Scholar]
- 11.Warrell, D. A., Molyneux, M. E., Beales, P. F. (1990) Trans. R. Soc. Trop. Med. Hyg. 84, Suppl. 2, 1–2.
- 12.Allen S J, O’Donnell A, Alexander N D E, Clegg J B. Q J Med. 1996;89:779–788. doi: 10.1093/qjmed/89.10.779. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard D I, Quinnel R J, Moustafa M, McKean P G, Slater A F, Raiko A, Dale D D, Keymer A E. Trans R Soc Trop Med Hyg. 1991;85:235–238. doi: 10.1016/0035-9203(91)90038-z. [DOI] [PubMed] [Google Scholar]
- 14.Al-Yaman F, Genton B, Mokela D, Narara A, Raiko A, Alpers M P. PNG Med J. 1996;39:16–22. [PubMed] [Google Scholar]
- 15.Old J M, Higgs D R. In: Methods in Haematology. Weatherall D J, editor. Edinburgh: Churchill Livingstone; 1983. pp. 74–102. [Google Scholar]
- 16.Jarolim P, Palek J, Amato D, Hassan K, Sapak P, Nurse G T, Rubin H L, Zhai S, Sahr K E, Liu S-C. Proc Natl Acad Sci USA. 1991;88:11022–11026. doi: 10.1073/pnas.88.24.11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlesselman J J. Case-Control Studies. Oxford: Oxford Univ. Press; 1982. pp. 207–213. [Google Scholar]
- 18.Ruwende C, Khoo S C, Snow R W, Yates S N R, Kwiatkowski D, Gupta S, Warn P, Allsopp C E M, Gilbert S C, Peschu N, Newbold C I, Greenwood B M, Marsh K, Hill A V S. Nature (London) 1995;376:246–249. doi: 10.1038/376246a0. [DOI] [PubMed] [Google Scholar]
- 19.Pasvol G, Weatherall D J, Wilson R J M. Nature (London) 1977;270:171–173. doi: 10.1038/270171a0. [DOI] [PubMed] [Google Scholar]
- 20.Willcox M, Bjorkman A, Brohult J, Pehrson P-O, Rombo L, Bengtsson E. Ann Trop Med Parasitol. 1983;77:239–246. doi: 10.1080/00034983.1983.11811704. [DOI] [PubMed] [Google Scholar]
- 21.Allen S, O’Donnell A, Alexander N. Lancet. 1996;348:1168–1169. doi: 10.1016/S0140-6736(05)65301-9. [DOI] [PubMed] [Google Scholar]
- 22.Genton B, Al-Yaman F, Mgone C S, Alexander N, Paniu M M, Alpers M P. Nature (London) 1995;378:564–565. doi: 10.1038/378564a0. [DOI] [PubMed] [Google Scholar]
- 23.Luzzi G A, Torii M, Aikawa M, Pasvol G. Brit J Haem. 1990;74:519–524. doi: 10.1111/j.1365-2141.1990.tb06344.x. [DOI] [PubMed] [Google Scholar]
- 24.Senok A C, Li K, Nelson E A S, Yu L M, Tian L P, Oppenheimer S J. Trans R Soc Trop Med Hyg. 1997;91:138–143. doi: 10.1016/s0035-9203(97)90200-5. [DOI] [PubMed] [Google Scholar]
- 25.Luzzi G A, Merry A H, Newbold C I, Marsh K, Pasvol G, Weatherall D J. J Exp Med. 1991;173:785–791. doi: 10.1084/jem.173.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuthavong Y, Butthep P, Bunyaratvej A, Fucharoen S, Khusmith S. Am J Clin Pathol. 1988;89:521–525. doi: 10.1093/ajcp/89.4.521. [DOI] [PubMed] [Google Scholar]
- 27.Oppenheimer S J, Hill A V S, Gibson D, Macfarlane S B, Moody J B, Pringle J. Trans R Soc Trop Med Hyg. 1987;81:322–326. doi: 10.1016/0035-9203(87)90253-7. [DOI] [PubMed] [Google Scholar]
- 28.Williams T N, Maitland K, Bennett S, Ganczakowski M, Peto T E A, Newbold C I, Bowden D K, Weatherall D J, Clegg J B. Nature (London) 1996;383:522–525. doi: 10.1038/383522a0. [DOI] [PubMed] [Google Scholar]
- 29.Giglioli G. Bull W H O. 1972;46:181–202. [PMC free article] [PubMed] [Google Scholar]
- 30.Greenwood B M, Greenwood A M, Bradley A K, Snow R W, Byass P, Hayes R J, N’Jie A B J. Lancet. 1988;i:1121–1127. doi: 10.1016/s0140-6736(88)91949-6. [DOI] [PubMed] [Google Scholar]
- 31.Greenwood B M. Ann Trop Med Parasitol. 1997;91:523–531. doi: 10.1080/00034989760897. [DOI] [PubMed] [Google Scholar]
- 32.Williamson W A, Greenwood B M. Lancet. 1978;i:1328–1329. doi: 10.1016/s0140-6736(78)92403-0. [DOI] [PubMed] [Google Scholar]
- 33.Kwiatkowski D, Bate C A W, Scragg I G, Beattie P, Udalova I, Knight J C. Ann Trop Med Parasitol. 1997;91:533–542. doi: 10.1080/00034989760905. [DOI] [PubMed] [Google Scholar]
- 34.Bone R C, Balk R A, Cerra F B, Dellinger R P, Fein A M, Knaus W A, Schein R M, Sibbald W J. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 35.Rubenstein M, Mulholland J H, Jeffery G M, Sheldon M W. Proc Soc Exp Biol Med. 1965;118:283–287. doi: 10.3181/00379727-118-29820. [DOI] [PubMed] [Google Scholar]
- 36.Shaheen S O, Aaby P, Hall A J, Barker D J P, Heyes C B, Shiell A W, Goudiaby A. Lancet. 1996;347:1792–1796. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 37.Shirakawa T, Enomoto T, Shimazo S, Hopkin J M. Science. 1997;275:77–79. doi: 10.1126/science.275.5296.77. [DOI] [PubMed] [Google Scholar]
- 38.Hill A V S. Trans R Soc Trop Med Hyg. 1992;86:225–226. doi: 10.1016/0035-9203(92)90282-h. [DOI] [PubMed] [Google Scholar]
- 39.Molineaux L. Lancet. 1997;349:1636–1637. doi: 10.1016/S0140-6736(97)22023-4. [DOI] [PubMed] [Google Scholar]
- 40.Molineaux L. Ann Trop Med Parasitol. 1997;91:811–825. doi: 10.1080/00034989760572. [DOI] [PubMed] [Google Scholar]