Abstract
This review covers the isolation, total synthesis, biologic activity, and more particularly the in vitro and in vivo antitumor activities of naturally occurring isocarbostyril alkaloids from the Amaryllidaceae family. Starting from these natural products, new derivatives have been synthesized to explore structure-activity relationships within the chemical class and to obtain potential candidates for preclinical development. This approach appears to be capable of providing novel promising anticancer agents.
Introduction
Natural products have played a highly significant role in the discovery and development of new drugs for the treatment of human diseases [1]. This is particularly evident in the cancer field, where more than 60% of drugs are of natural origin [2]. Plants from the Amaryllidaceae family have long been known for their medicinal and toxic properties. Indeed even in ancient Greece, the oil from Narcissus species was already being used successfully for the treatment of cancer [3]. Consequently, efforts have been made to isolate the active ingredients responsible for this antitumor activity. Some 48 alkaloids bearing a variety of carbon skeletons have been isolated from Narcissus species [4]. One small group of these alkaloids does not contain basic nitrogen atoms and is represented by hydroxylated benzophenanthridone [5] or isoquinolinone types of structure. The most widely known compounds [5] of this group are narciclasine 1, lycoricidine 2, and pancratistatin 3 (Figure 1), and the most frequently used term in the literature to describe this group of alkaloids is the isocarbostyrils [6,7].
Figure 1.
Chemical structures of narciclasine, lycoricidine and pancratistatin.
All these natural products have demonstrated potent in vitro cytotoxicity against cancer cell lines [7] and potent in vivo antitumor activity against mouse M-5076 sarcoma and P388 leukemia [7]. Therefore, this family as a whole seems of interest as a potential source of new lead structures for the development of a future generation of anticancer drugs.
This review focuses on the isocarbostyril alkaloid family, with a special emphasis on the isolation and synthesis of these natural products and their hemi- and fully synthetic derivatives and their in vitro and in vivo antitumor activity. Finally, recent advances in understanding the mechanism of action of these antitumoral molecules as well as their original role in the control of the plant growth will be discussed.
Isolation of Amaryllidaceae Isocarbostyril Alkaloids
Narciclasine and Natural Derivatives
Narciclasine 1, also known as lycoricidinol given it is the 7-hydroxy derivative of lycoricidine 2 (Figure 1), was the first alkaloid of the class isolated in 1967 from Narcissus (Amaryllidaceae species) bulbs based on its inhibition of the growth of wheat grain radicles [8]. The typical procedure for the isolation of this compound involves ethanolic extraction of fresh daffodil bulbs. The alcohol is then evaporated to leave an aqueous residue, which is itself extracted with dichloromethane to remove nonpolar contaminants. Narciclasine is then extracted from the residual aqueous phase with ethyl acetate. After evaporation of the solvent, the residue is dissolved in ethanol, and after that, solid deposit was further purified by column chromatography [8,9]. Narciclasine is obtained as a pure product after alcohol or acetic acid crystallization (as light yellowish needles) of the eluted fraction containing the compound. The structure of narciclasine was elucidated in 1970 by 1H nuclear magnetic resonance (NMR) and 13C NMR, mass spectral, infrared, and elemental analyses and was further characterized with respect to optical rotation [9,10].
One of the most widely reported sources of narciclasine is the bulbs of Narcissus species [4,8,11]. Thirty-two species and varieties of this genus have been examined for narciclasine content, which varies from 30 to 200 mg/kg fresh bulbs. The typical content in this genus is 100 to 120 mg/kg (Table 1) and may vary during the year. For example, a content of 200 mg/kg was exceptionally found in bulbs of Narcissus incomparabilis Mill. Var. Helios at the flowering stage in March (Table 1). The occurrence of narciclasine was also investigated in others species of Amaryllidaceae, including Lycoris radiata [12,13], Haemanthus [6,11], Hymenocallis [7,11,14,15], Brachystola magna [16], Galanthus [11], Leucojum [11], Pancratium maritimum [11], Sprekelia formosissima [11], Sternbergia lutea [11], and Vallota speciosa [11]. The content of narciclasine in these species does not exceed 50 mg/kg fresh bulbs (Table 1). Narciclasine was exceptionally found in high concentrations (∼135 mg/kg) in Zephyranthes flava, a tropical and subtropical plant cultivated in India (Table 1) [6].
Table 1.
Occurrence of Narciclasine 1, Lycoricidine 2, and Pancratistatin 3 (in mg/kg of Bulbs or Seeds) in Some Amaryllidaceae Species.
| Amaryllidaceae Species | Compounds | ||
| 1 | 2 | 3 | |
| N. imcomparabilis | 200 in March | NI | NI |
| 100–120 in November | |||
| Narcissus “Carlton” | 100–120 | NI | NI |
| H. kalbreyeri | 45 | 17 | 157 |
| Z. flava | 135 | NI | 165 |
| P. maritimum | 50 | NI | 6 |
| L. radiata | 4.2 | 3.2 | NI |
| Ismene x “Sulfur Queen” | NI | 32 | NI |
| H. littoralis | 5 | 118 | 100–150 from Hawaii 22 from Arizona |
| B. magna | 1.5 | NI | 2.5 |
| Z. carinata | NI | NI | 7.5 |
NI indicates not isolated.
However, in general and based on analyses to date, Narcissus bulbs are the best and easiest to obtain source of narciclasine, given that these plants are relatively common and can grow in many countries with different climates.
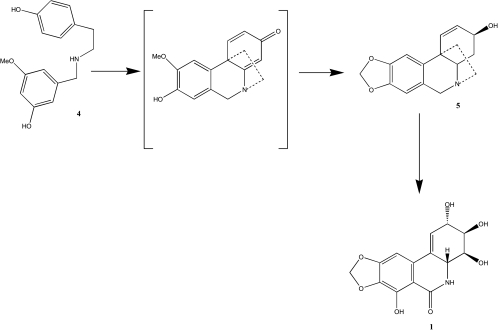
The biosynthesis of these Amaryllidaceae alkaloids in plants was deciphered in 1971 [17]. Narciclasine is synthesized from O-methylnorbelladine 4 by para-para phenol coupling to obtain vittatine 5 as an intermediate (Scheme 1) [17,18]. Subsequent elimination of two carbon atoms and hydroxylations of compound 5 then leads to narciclasine (Scheme 1).
Scheme 1.
The biosynthesis of narciclasine.
In 1985, the first biosynthetic derivative of narciclasine, compound 6 (Figure 2), was isolated in good quantities from Haemanthus kalbreyeri [19] and Z. flava [20] (concentrations of 78 and 165 mg/kg, respectively), two tropical and subtropical Amaryllidaceae species. So this natural product was named kalbreclasine, and it is the 2-O-β-d-glucopyranoside derivative of narciclasine. This compound, whose structure was fully elucidated by spectroscopic and chemical methods, provoked a significant activation of splenic lymphocytes in vitro, a feature characteristic of immunostimulants [19]. Another β-d-glucoside derivative of narciclasine 7 (Figure 2) was isolated in 1991 in trace amounts from P. maritimum (∼3.5 mg/kg) [21]. Currently, the last known natural derivative of narciclasine (Figure 2) isolated is trans-dihydronarciclasine 8, which is extracted at low levels (11 mg/kg) from Zephyranthes candida [22].
Figure 2.
Natural derivatives of narciclasine.
Lycoricidine and Natural Derivatives
7-Deoxynarciclasine 2 (Figure 1), more generally known as lycoricidine, was first discovered in L. radiata in 1968 [12]. The product was isolated from methanol extracts of the plant's bulbs by a similar procedure to that used for narciclasine. Lycoricidine, which was obtained only at low levels (∼3 mg/kg; Table 1), was found similar to narciclasine to be a plant growth inhibitor [12]. The structure of lycoricidine was fully established particularly by 1H NMR and 13C NMR and by chemical reactions [12,23]. Lycoricidine and narciclasine were shown in 1983 to have a potent insecticidal action, in addition to reducing feeding activity in the larvae of certain butterfly species [13].
Lycoricidine was also isolated in low quantities (∼17 mg/kg) from H. kalbreyeri [6] and in moderate quantities (∼32 mg/kg) from Ismene x “Sulphur Queen” [15]. The best sources of lycoricidine are bulbs of Hymenocallis littoralis collected from the wild in Hawaii as these yield in the order of 118 mg/kg [14,24]. However, if this rare tropical plant is cultivated in Arizona, the content of lycoricidine is much reduced, varies during the year, and only a maximum of 15 mg/kg can be isolated even in the peak month of October [14]. This difference in the content to the native Hawaiian bulb could be explained by an Arizona climate that is much hotter, drier, and sunnier [14].
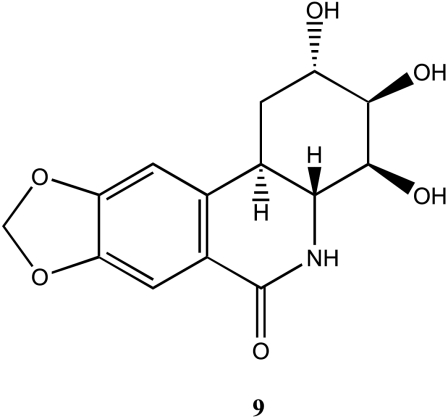
To date, only one natural derivative of lycoricidine 9 (Figure 3) has been isolated from H. littoralis [14,23,25] (the tropical spider lily) and Hymenocallis caribaea [7]. Compound 9 (7-deoxy-trans-dihydronarciclasine or alternatively trans-dihydro lycoricidine) is a trans-dihydro derivative of lycoricidine in which the two ring cycles are trans linked. Hymenocallis littoralis contains a maximum of 16 mg/kg of 9 when sampled in October [14], whereas H. caribaea contains a maximum of only 3.8 mg/kg. The structure has been fully elucidated by spectroscopic and chemical methods [7,25].
Figure 3.
Chemical structure of the only one isolated natural derivative of lycoricidine.
Pancratistatin and Natural Derivatives
In 1984, pancratistatin 3 (Figure 1) was first discovered in a Hawaiian species originally designated as Pancratium littorale, but later reclassified as H. littoralis [26]. The bulb section of this native Hawaiian plant was extracted with dichloromethane-methanol-water and the extract was concentrated to obtain an aqueous phase. Organic compounds were extracted with n-butanol and purification of the resulting crude product was undertaken using gel permeation chromatography (Sephadex LH-20) and precipitation/crystallization to give the pure natural product 3 [26]. This experimental procedure is very effective for laboratory-scale isolation of pancratistatin, but for largescale production, the cost is too high. Another procedure was then developed to avoid the use of gel permeation, but this approach remains more complicated than the isolation of narciclasine [14]. The structure of pancratistatin was fully elucidated by 1H NMR and 13C NMR, mass spectrometry, infrared, and elemental analyses [24,26] and was corroborated by an X-ray crystal structure determination of the hemisynthesized 7-methoxy derivative [26]. As with narciclasine and lycoricidine, pancratistatin has been found to exhibit strong in vitro RNA antiviral activity [27]. In vivo, pancratistatin increased survival by 100% when it was used to treat mice infected with Japanese encephalitis [27]. Furthermore, this product showed activity against two other RNA-containing flaviviruses and bunyaviruses, namely Punta Toro and Rift Valley fever [27]. Pancratistatin and lycoricidine also showed an in vitro antiparasitic effect against a microsporidium causing infections in humans [28].
The rare tropical bulbs of H. littoralis seem to be a good source of pancratistatin as it can be isolated from these in the order of 100 to 150 mg/kg when bulbs are obtained from the wild in Hawaii (Table 1) [14,24]. However, the compound has to be commercially extracted from field- and greenhouse-grown bulbs or from tissue cultures cultivated, for example, in Arizona, which generate lower levels of pancratistatin (a maximum of 22 mg/kg) even in the peak month of October (Table 1) [14]. After October, when the bulb becomes dormant, levels of pancratistatin drop to only 4 mg/kg by May [14]. Field-grown bulbs, which show monthly changes in pancratistatin content, generate somewhat smaller amounts (2–5 mg/kg) compared to those grown in greenhouse cultivated over the same period [14]. The occurrence of pancratistatin has also been investigated in other Amaryllidaceae, including several species of Hymenocallis [15], B. magna [16], and P. maritimum [15]. The pancratistatin content of these species does not exceed 29 mg/kg of fresh bulbs. Pancratistatin was exceptionally found in good quantity in Z. flava [20] and H. kalbreyeri [6] (Table 1), two tropical and subtropical plants cultivated in India. Consequently and to date, the best sources of pancratistatin are rare, tropical, and subtropical bulbs of Z. flava, H. kalbreyeri, and H. littoralis when these plants are cultivated in their native region.
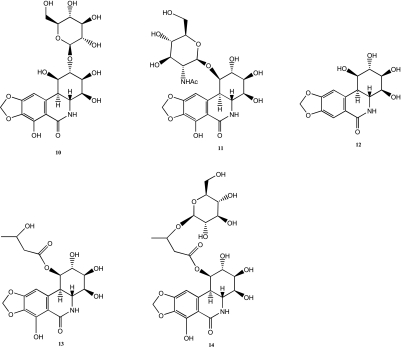
The first natural derivative of pancratistatin, compound 10 (Figure 4), was isolated from H. kalbreyeri in 1989 [6]. This 2-O-β-d-glucopyranoside derivative, pancratiside, was found to promote the germination of seeds and the growth of roots similar to kalbreclasine 6 but unlike its aglycone derivatives [6]. 7-Deoxy-pancratistatin 12 (Figure 4) was also discovered in the same plant in small amounts (∼30 mg/kg) [6]. The structures of these two alkaloids were established by comprehensive spectral analyses and chemical transformations [6]. Another glycoside derivative, telastaside 11 (Figure 4), was also isolated from this family of plants [29]. In 1998, two new derivatives of pancratistatin 13 and 14 (Figure 4) were extracted in moderate amounts (24 and 44 mg/kg, respectively) from Zephyranthes carinata [30]. Key substituents in these two molecules were identified as a 3-hydroxy-butyryl group for compound 13 and as a 3-O-glucosylated butyryl group for derivative 14 [30]. The structures of these two natural products were established by 1D and 2D NMR spectral analyses [30].
Figure 4.
Chemical structures of natural derivatives of pancratistatin.
Synthesis of Isocarbostyril Alkaloids
In general, these alkaloids are isolated only in moderate quantities from natural sources. As they have revealed promising antitumor activities (Sections 4 and 5), there is a strong need to develop total syntheses of these alkaloids and their derivatives to obtain them more easily and in appreciable quantities. There are more than 25 references on this subject revealing these syntheses to be complex multistep processes. The full synthesis of these alkaloids has been reviewed on several occasions [31,32], and the focus here will be on representative syntheses for narciclasine, lycoricidine, and pancratistatin.
Narciclasine and Lycoricidine
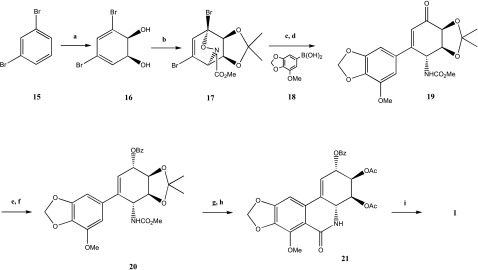
One of the shortest synthetic routes for narciclasine and lycoricidine was established by Rinner and Hudlicky [31] and Hudlicky et al. [32]. The key product of this synthesis was the cyclohexadiene diol 16 (Scheme 2) obtained by the whole-cell fermentation of 1,3-dibromobenzene 15 with recombinant Escherichia coli JM0109 [33]. This diol 16 was then protected by an acetonide group, and the resulting product subject to a hetero-Diels-Alder reaction to generate oxazine 17. A Suzuki coupling of one of the bromines on 17 with the boronic acid derivative 18 afforded the desired coupled product, which was treated with a silylsilane derivative to form the ketone 19. The reduction of the ketone function was followed by a Mitsunobu reaction to obtain benzoate 20. After replacement of the acetonide by acetates, a modified Bischler-Napieralski ring-closure gave the protected product 21. Cleavage of the phenolic methyl function and removal of protecting groups afforded narciclasine 1 in 12 steps with an overall yield of 0.5% from the cyclohexadiene diol 16. The synthesis of lycoricidine 2 was realized in nine steps (30% overall yield) by the same method using a cyclohexadiene diol such as 16 obtained by biooxidation with the same bacteria E. coli [34].
Scheme 2.
a: E. coli JM109 (pDTG601A), 4 g/L. b: i) DMP, acetone, p-TsOH, r.t.; ii) NHCO2Me, NaIO4, r.t., 70%. c: borate 18, Pd(PPh3)4, aq Na2CO3, PhH, reflux, 30%. d: TTMSS, AIBN, PhH, reflux, 80%. e: NaBH4, CeCl3, MeOH, 0°C, 80%. f: BzOH, Bu3P, DEAD, THF, r.t., 65%. g: i) Dowex 50X8-100, MeOH, r.t.; ii) Ac2O, pyridine, DMAP, r.t., 70%. h: Tf2O, DMAP, CH2Cl2, 0°C, 40%. i: i) Amberlyst A21, MeOH; ii) LiCl, DMF, 120°C, 20%.
A second method of synthesis used for narciclasine and lycoricidine was established in 1999 by Keck as reviewed in the work of Rinner and Hudlicky [31]. The starting material of these total syntheses is the commercially available d-gulonolactone 22 that fits correctly with three of the four stereocenters of these isocarbostyril products (Scheme 3). After 2,3-acetonide protection, compound 23 was obtained in two steps. Oxidation of 23 followed by a Corey-Fuchs reaction of this latter product gave dibromide 24. The lactone function of 24 was then converted to the O-benzyl amine derivative 25. A Pd-catalyzed coupling reaction of 25 with compound 26 provided the alkyne 27. The radical cyclization of this latter product afforded compound 28 with an 88% yield. Cleavage of the tosyl group in 28 and methylation of the phenolic hydroxyl functionality allowed aluminum-catalyzed cyclization to deliver the protected product 29. Deprotection of the thioether and removal of acetonide by acid treatment afforded narciclasine 1 in 14 steps from d-gulonolactone with an overall yield of 16%. This method of synthesis was also used for lycoricidine in 11 steps with an overall yield of 27% starting from D-gulonolactone 22 and the appropriate aromatic analog of 26 [31].
Scheme 3.
a: DMP, p-TsOH, DMF, 79%. b: AcOH, H2O, THF, 79%. c: NaIO4, CH2Cl2. d: CBr4, PPh3, Et3N, 80% (more than two steps). e: l-Selectride, Et2O, -78°C. f: HCl·H2NOBn, pyridine, 90% (more than two steps). g: n-BuLi, Et2O, -90°C, 93%. h: Pd(OAc)2, PPh3, CuI, Et3N, 26, THF, 89%. i: PhSH, hv, toluene, 27°C, 88%. j: SmI2, THF, H2O, 0°C, 94%. k: MeI, K2CO3, DMF, 96%. l: Me3Al, THF, -15°C to 65°C, 72%. m: SmI2, MeOH, THF, 0°C, 87%. n: TFA, 0°C, 89%.
Pancratistatin
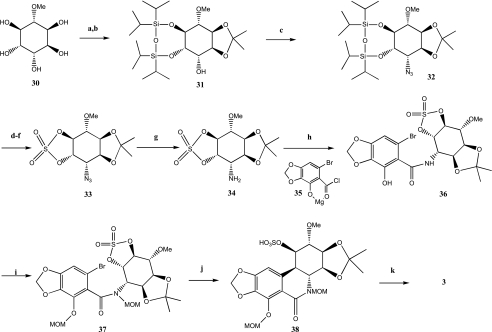
Danishefsky et al., as reviewed in the work of Rinner and Hudlicky [31], reported in 1989 the first total synthesis of racemic pancratistatin. Thereafter, numerous research groups published the stereoselective synthesis of pancratistatin. These syntheses were previously reviewed [31] and include those of Tian et al. [35], Doyle et al. [36], Trost and Pulley [37], Rigby et al. [38], Magnus and Sebhat [39], and Ko et al. [40]. Another interesting synthesis of pancratistatin was reported in 2006 by Li et al. [41] and started from pinitol 30 whose stereogenic centers exactly match those of the C-ring of pancratistatin (Scheme 4). Protection of the diol functions of compound 30 gave compound 31. The free hydroxyl of this was subsequently substituted by an azide to give 32. After removal of the silyl function, a cyclic sulfate was installed to obtain product 33. The Staudinger reaction gave the free amine 34 from azide 33. The coupling reaction between 34 and 35 gave compound 36 with a moderate yield. Methocymethyl protection of both the amide and the free phenol gave compound 37. Treatment of this latter product with t-BuLi followed by addition of cerium chloride gave compound 38. Full deprotection of 38 by BBr3 and methanol afforded pancratistatin 3 in 12 steps from commercially available pinitol with an overall yield of 2.3%.
Scheme 4.
a: TIPDSCl2, imidazole, DMAP, DMF, 24%. b: DMP, p-TsOH, acetone, 81%. c: PPh3, DEAD, CH3SO3H, CH2Cl2, 0°C to r.t. then NaN3, DMF, 60°C, 72%. d: TBAF, THF, 0°C to r.t., 100%. e: SOCl2, Et3N, CH2Cl2, 0°C. f: NaIO4, RuCl3, aq CH3CN, 87% (more than two steps). g: PPh3, aq THF, 0°C to r.t., 94%. h: Et2O, 35, 0°C, 64%. i: K2CO3, MOMCl,DMF, 84%. j: t-BuLi, CeCl3, ultrasound, THF, -78°C to r.t., 72%. k: BBr3, CH2Cl2, -78°C to 0°C, 1 hour then MeOH, -78°C to 0°C, 2 hours, 52%.
Given the higher availability of narciclasine in extracts of Narcissus species (up to 200 mg/kg), Pettit et al. [42,43] have developed and patented a second process to synthesize pancratistatin from Amaryllidaceaederived narciclasine. The hydroxyl functions of narciclasine are first protected and the olefin function oxidized to give epoxide 39 (Scheme 5). Hydrogenation of this latter product and subsequent saponification yields a mixture of four compounds, one of which is the desired diol 40. Activation of this diol with thionyl chloride and oxidation of the epimeric sulfites give the cyclic sulfate 41. Nucleophilic opening of compound 41 and hydrolysis of the sulfate and acetonide functions affords the benzoate product 42. Deprotection of the benzoyl group at C-1 position gives pancratistatin 3 in 10 steps and an overall yield of 3.6% from narciclasine 1.
Scheme 5.
a: DMF, DMP, p-TsOH, 97%. b: Ac2O, pyridine, 81%. c: m-CPBA, phosphate buffer, CH2Cl2, H2O, 52%. d: i) H2, Pd/C 10%; ii) K2CO3, MeOH, H2O, 28%. e: SOCl2, Et3N, THF; f: RuCl3·3H2O, NaIO4, MeCN, CCl4, H2O, 47% (more than two steps). g: i) PhCO2H, CsCO3, DMF; ii) THF, H2O, H2SO4, 74%. h: K2CO3, MeOH, 75%.
In Vitro Antitumor Activity
Narciclasine, Lycoricidine, Pancratistatin, and Natural Derivatives
The cytotoxicity of these isocarbostyril alkaloids was first demonstrated in basic assays some 30 years ago [12,44] and later confirmed in standard MTT colorimetric assays on diverse human tumor cell lines by the National Cancer Institute (NCI) [7,23,45,46].
Narciclasine 1 exhibits in vitro potent cytotoxicity against human cancer cell lines and murine P388 leukemia cells (Table 2) [45,46]. The cytotoxicity of this product was evaluated in 60 cancer cell lines by the NCI, and the mean IC50 value (the concentration which inhibits overall cell growth in culture by 50%) was 0.046 µM [45]. With respect to narciclasine, lycoricidine 2 demonstrates cytotoxic activity against these 60 cancer cell lines, which is ∼10 times weaker (mean IC50 = 0.33 µM), and pancratistatin 3 an activity µ5 times weaker (IC50 = 0.26 µM) [46]. The observed cytotoxicity of these three isocarbostyrils against murine P388 leukemia cell line is similar but results against human cancer cell lines (Table 2) confirm those found by the NCI for lycoricidine but not for pancratistatin [23,45]. Against the murine P388 leukemia cell line, dehydronarciclasine 8 showed more potent cytotoxicity than narciclasine (Table 2), but in the 60 cancer cell line panel, the cytotoxicity was of the same order of magnitude with a mean IC50 of 0.053 µM [23,45,46]. Dehydrolycoricidine 9 revealed the same level of cytotoxicity as lycoricidine (Table 2) [23]. These results with compounds 8 and 9 revealed that the double bond between the C-1 and C-10b is not essential for the cytotoxicity of these isocarbostyril alkaloids. For glycoside derivatives 6, 7, 10, and 11 (Figures 2 and 4), there are no data in the literature on the cytotoxicity measured by MTTassays against cancer cells. However, for glucoside derivative 7, a potato disc cytotoxicity assay has revealed an activity very similar to narciclasine [21]. For 7-deoxy pancratistatin 12, only two cancer cell lines have been tested and this product demonstrated a 10- to 20-fold lower cytotoxic activity than pancratistatin (Table 2) [47]. It can be concluded that the phenolic hydroxyl group of compounds 1 and 3 contributes to the greater (≥10-fold) activity of these products compared to that of their congeners 12 and 2.
Table 2.
In Vitro Cytotoxic-Related Antitumor Effects (IC50 Values in µM)* of Certain Naturally Occurring Isocarbostyril Alkaloids [23,44].
| Compounds | Cancer Cell Lines | ||||||
| Leukemia P388 | Pancreas BXPC-3 | Breast MCF-7 | CNS SF268 | Lung-NSC NCI-H460 | Colon KM20L2 | Prostate Du-145 | |
| 1 | 0.042 | 0.011 | 0.010 | 0.010 | 0.027 | 0.011 | 0.011 |
| 2 | 0.065 | 0.24 | 0.16 | 0.41 | 0.18 | 0.29 | 0.17 |
| 3 | 0.052 | 0.061 | 0.071 | 0.043 | 0.098 | 0.077 | 0.046 |
| 8 | 0.0078 | 0.039 | 0.017 | 0.065 | 0.030 | 0.049 | 0.021 |
| 9 | 0.099 | 0.16 | 0.12 | 0.20 | 0.15 | 0.17 | 0.14 |
| 12 | 1.42 | NT | NT | NT | NT | 0.71 | NT |
Data are initially given in µg/ml [50% effective dose (ED50) or 50% growth inhibition (GI50)].
NT indicates not tested.
The hydroxybutyril derivative of pancratistatin 13 gave two-fold better cytotoxicity than pancratistatin against P388 leukemia cells [30]. This better activity was then confirmed by the NCI in the 60-cancer cell line panel with a mean IC50 of 0.037 µM [46]. The glucoside derivative 14 revealed the same order of cytotoxicity as pancratistatin against P388 leukemia cells [30].
Fully and Hemisynthesized Derivatives
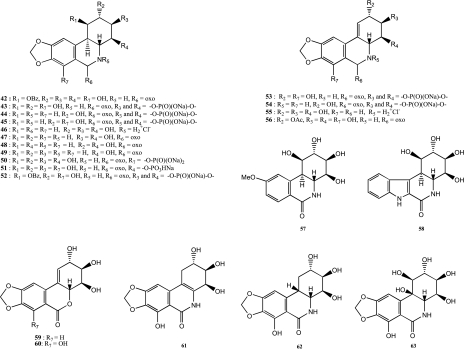
A series of derivatives and analogs have been synthesized by full or hemisynthesis to obtain more active and/or soluble products (Figure 5) that could be potentially selected for preclinical development as anticancer agents.
Figure 5.
Chemical structures of fully and hemisynthesized isocarbostyril derivatives.
Only one compound that has been synthesized, a derivative of narciclasine (Scheme 5) [42,43], has been found to be more active than pancratistatin. This 1-benzoate pancratistatin derivative 42, phenpanstatin, has revealed a cytotoxicity 10 times higher than pancratistatin 3 against a panel of human cancer cell lines (Tables 2 and 3) [45].
Table 3.
In Vitro Cytotoxic-Related Antitumor Effects (IC50 Values in µM)* of Fully and Hemisynthetic Isocarbostyril Alkaloid Derivatives.
| Compounds | Cancer Cell Lines | ||||||
| Leukemia P388 | Pancreas BXPC-3 | Breast MCF-7 | CNS SF268 | Lung-NSC NCI-H460 | Colon KM20L2 | Prostate Du-145 | |
| 42 | 0.0037 | 0.0044 | 0.00072 | 0.0013 | 0.00023 | 0.00086 | 0.00049 |
| 43 | 8.1 | 8.1 | 7.1 | 7.1 | 9.3 | 9 | 5.6 |
| 44 | 4.2 | > 26.5 | 19.1 | > 26.5 | > 26.5 | > 26.5 | > 26.5 |
| 45 | 2.2 | 14.2 | 11.7 | 22.4 | 19.8 | 24.4 | 13.2 |
| 46 | 10.5 | 4.4 | 5.4 | 5.1 | 2.5 | 5.4 | 2.2 |
| 47 | 5 | > 36.1 | > 36.1 | > 36.1 | > 36.1 | > 36.1 | > 36.1 |
| 48 | 1.6 | NT | NT | NT | NT | NT | NT |
| 49 | 153.6 | NT | NT | NT | NT | NT | NT |
| 50 | 0.53 | 0.44 | 0.44 | 0.18 | 0.42 | 0.38 | 0.058 |
| 51 | 0.042 | 0.42 | 0.42 | 0.28 | 0.89 | 0.56 | 2.2 |
| 52 | 0.12 | 0.49 | 0.08 | 0.33 | 0.056 | 0.25 | 0.25 |
| 53 | 0.031 | 0.18 | 0.15 | 0.12 | 0.15 | 0.15 | 0.08 |
| 54 | 4.5 | 14.1 | 10.7 | 16.8 | 12.5 | 14.9 | 10.4 |
| 55 | 22.6 | 14 | 10.8 | 9.2 | 10.8 | > 32 | > 10.2 |
| 56 | < 0.03 | 0.78 | 0.72 | 0.57 | 0.96 | 0.54 | 0.78 |
| 57 | 14.6 | 16.6 | 14.9 | 11.2 | 9.5 | 12.2 | 8.8 |
| 58 | 60.2 | > 32.9 | > 32.9 | NT | NT | > 32.9 | NT |
Data are initially given in µg/ml (ED50 or GI50).
NT indicates not tested.
Naturally occurring isocarbostyril alkaloids such as pancratistatin have low aqueous solubility (∼50 µg/ml) [45]. This has limited their clinical evaluation in intravenous formulations. To increase aqueous solubility, phosphate prodrugs, which release the active drug through the action of cellular phosphatases, have been synthesized [23,45,48]. Several positions (4-OH, 7-OH, and 3,4-OH) on the natural isocarbostyril alkaloids were used to obtain 3,4-cyclic phosphate derivatives 43, 44, 45, 50 (7-O-phosphate pancratistatin), 51 (4-O-phosphate pancratistatin), 52, 53 (narcistatin), and 54 (Figure 5) [23,45,48]. These phosphate derivatives had considerably improved aqueous solubility, notably the sodium salt of narcistatin 53 (60 mg/ml) and have been duly patented [49–52]. All phosphate derivatives have markedly lower cytotoxicity in vitro compared to their active parent compound (Tables 2 and 3) [23,45,48]. However, as indicated above, hydrolysis of the phosphate groups to release the active isocarbostyril is expected to be very effective in vivo. In vivo antitumor evaluations will be undertaken shortly to validate this concept of water-soluble phosphate prodrugs [23].
To explore the structure-activity relationship (Figure 5 and Tables 2 and 3) of these isocarbostyril alkaloids, several other key positions in the molecule were modified. One modification involved the reduction of the lactam carbonyl group. For this purpose, 2-acetate lycoricidine 56 (Figure 5) was synthesized as an intermediate, but this modification was found to have a low impact on cytotoxicity. In contrast, compounds 46 and 55 (Figure 5) were found to be inactive against the panel of human cancer cell lines (Table 3) [25]. Fully synthetic compounds 59 and 60 (Figure 5) in which the lactam carbonyl was also replaced by a lactone function, which is considered an analog [5], showed no significant cytotoxic activity against L1210 murine leukemia cells [5]. All these modulations appear to indicate that the lactam carbonyl function is essential for cytotoxic activity.
Three deoxy-analogs of these isocarbostyril alkaloids 47, 48, and 49 (Figure 5) were synthesized to determine the minimum structural pharmacophore [53,54]. These three analogs showed weak antitumor activity (Table 3) and so revealed that the C-2, C-3, and C-4 hydroxyl groups were essential for the cytotoxic activity and can be considered as a minimum pharmacophore [53,54].
Another modulation was the B/C ring junction stereochemistry (Figure 1) and was evaluated through the synthesis of derivatives 61, 62, and 63 (Figure 5) [7,55]. For the isonarciclasine 61, the B/C ring junction is planar because a double bond connects the two rings. A cis-B/C ring junction was obtained in products 62 and 63 after hydrogenation or oxidation of the double bond of narciclasine [7,22]. All these products showed weaker cytotoxicity than the parent natural compounds. Compound 61 gave an IC50 in the order of 12 µM and 62 an IC50 of ∼4 µM [7]. The cytotoxicity of compound 63 against P388 murine leukemia and several human cancer cell lines was found to be about 1000 times less potent than pancratistatin [55]. All these results appear to confirm the need for a trans-B/C ring junction in these compounds to maintain potent cytotoxicity.
Modulation of the benzodioxole moiety of pancratistatin was investigated through the full synthesis of analogs 57 and 58 (Figure 5) [47,54]. The methoxy analog 57 and the β-carboline-1-one derivative 58 were revealed to be 100-fold less cytotoxic than the corresponding natural product [47,54]. These results show that an intact methylenedioxyphenyl or benzodioxole functionality is essential for significant cancer cell cytotoxicity in this class of natural compounds.
In Vivo Antitumor Activity
Pancratistatin and narciclasine have proven to be effective in murine P388 lymphocytic leukemia and M-5076 ovary sarcoma models in vivo [24]. Table 4 (A and B) shows the in vivo survival values from the NCI database [46] for the antitumor activities of pancratistatin (A) and narciclasine (B) in different mouse models. The T/C index represents the percentage survival ratio of drug-treated (T) compared to untreated (C) mice. A T/C value >125% indicates that drug treatment significantly enhances the survival of the tumor-bearing mice.
Table 4.
National Cancer Institute Pancratistatin (A) and Narciclasine (B) In Vivo Antitumor Activities in a Range of Different Mouse Tumor Models.
| (A) Pancratistatin, mg/kg | |||||
| T/C Survival | B16 Melanoma | L1210 Leukemia | P388 Leukemia | M5076 Sarcoma | M5076 Sarcoma |
| Tumor Site Inoculation | i.p. | i.p. | i.p. | i.p. | s.c. |
| 0.18 | NT | NT | 113% | 153% | 101% |
| 0.25 | NT | NT | NT | NT | 106% |
| 0.37 | NT | NT | 122% | NT | NT |
| 0.38 | NT | 120% | 119% | NT | NT |
| 0.5 | NT | NT | NT | NT | 112% |
| 0.75 | NT | 122% | 139% | 159% | 106% |
| 0.78 | NT | NT | 133% | 132% | NT |
| 1 | NT | NT | NT | NT | 109% |
| 1.5 | 100% | 121% | 142% | 166% | 111% |
| 1.56 | 108% | 131% | 144% | 129% | NT |
| 2 | NT | NT | NT | NT | 112% |
| 3 | 103% | 133% | 161% | 184% | 125% |
| 3.12 | 129% | 131% | 151% | 143% | NT |
| 4 | NT | NT | NT | NT | 113% |
| 6 | 114% | NT | 165% | 177% | 126% |
| 6.25 | 110% | NT | 173% | 152% | NT |
| 8 | NT | NT | NT | NT | 121% |
| 12 | NT | NT | 156% | 144% | NT |
| 12.5 | NT | NT | 203% | NT | NT |
| 24 | NT | NT | NT | Toxic | NT |
| 25 | Toxic | Toxic | Toxic | NT | NT |
| (B) Narciclasine, mg/kg | |||||
| T/C Survival | B16 Melanoma | L1210 Leukemia | P388 Leukemia | M5076 Sarcoma | Lewis Lung Carcinoma |
| Site of Inoculation | i.p. | i.p. | i.p. | i.p. | i.v. |
| 0.62 | 115% | 110% | 110% | NT | 93% |
| 1.25 | 111% | 108% | 129% | NT | 109% |
| 2.5 | 109% | 113% | 162% | NT | 117% |
| 3 | NT | NT | NT | 133% | NT |
| 5 | 123% | 115% | 100% | NT | NT |
| 6 | NT | NT | NT | 160% | NT |
| 10 | NT | NT | 87% | NT | NT |
| 12.5 | NT | NT | NT | NT | NT |
All compounds were administrated intraperitoneally.
i.p. indicates intraperitoneal; i.v., intravenous; NT, not tested; s.c., subcutaneous.
The promising antineoplastic activity of pancratistatin has led to its preclinical development and selection as an important synthetic starting point for a growing number of research groups. Pancratistatin is particularly attractive for clinical development because of its potent activity against experimental melanoma and ovary carcinoma. Unfortunately, pancratistatin exhibits very low water solubility (53 µg/ml) and this property has complicated its formulation for intravenous administration. Although the solubility of this isocarbostyril can be increased in organic solvents, such as dimethylformamide and dimethylsulfoxide, and lower boiling aliphatic alcohols, their use as formulation components is not desirable.
Mechanism of Action
Little is known regarding the anticancer mechanism of action of this family of compounds. Narciclasine and lycoricidine were first identified as plant growth inhibitors [8,12]. In plants, there is clear evidence that narciclasine inhibits the synthesis of proteins and the development of chloroplasts. Additionally, the compound isolated from the mucilage of Narcissus bulbs showed inhibitory effects on the growth and plastid development in excised radish cotyledons [56]. Narciclasine (0.1 µM) starts to show inhibitory effects on plant isocitrate lyase and hydroxypyruvate reductase activities after 24 hours of incubation in light. When the concentration is increased to 10 µM, the activities of both enzymes are completely inhibited [56]. From ultrastructure studies, 1 µM narciclasine markedly prevents the degradation of protein bodies and lipid bodies, as well as totally preventing chloroplast formation in excised radish cotyledons [56]. Visible symptoms of tepal senescence in cut Iris x “Hollandica” (cv. Blue Magic) flowers are delayed by placing one cut daffodil flower (Narcissus pseudonarcissus, cv. “Carlton”) in the same vase [57]. Addition of mucilage, exuded by daffodil stems to the vase water has the same effect as the flowering daffodil stem. The active compound in the mucilage was identified as narciclasine. This delay of senescence by narciclasine correlates with a delayed increase in protease activity and marked reduction in maximum protease activity [57]. Narciclasine, although not affecting in vitro protease activity, is known to inhibit protein synthesis at the ribosomal level. Narciclasine was also originally described as an antimitotic substance displaying colchicine-like effects [8]. It was also found to be an inhibitor of peptide bond formation in eukaryotic ribosomes given its ability to bind to the 60-S ribosomal subunit and more precisely to the peptidyltransferase center [58,59]. Furthermore, unlike many other anticancer drugs, narciclasine has been found not to interact or form a complex with DNA [60]. More recently, McLachlan et al. [61] demonstrated that pancratistatin, whose chemical structure is very close to that of narciclasine, induced rapid apoptosis in neuroblastoma cells accompanied by disruption of the mitochondrial membrane potential. Additionally, a decrease in ATP synthesis and an increase in the production of reactive oxygen species, indicative of a dysfunction of the mitochondrial respiratory chain, were observed in intact mitochondria incubated with the molecule [61]. These changes were not observed in normal fibroblasts [61,62] and normal endothelial cells [63]. Caspase-3 activation and exposure of phosphatidyl serine on the outer leaflet of the plasma membrane were earlier events than the generation of reactive oxygen species and DNA fragmentation observed following pancratistatin treatment of cancer cells [64]. By investigating isolated mitochondria, McLachlan et al. [61] demonstrated that pancratistatin acted directly on mitochondria and confirmed that this effect was selective for mitochondria isolated from cancer cells. Quite recently, Griffin et al. [62] undertook a synthetic structure-activity relationship evaluation, which indicated that the minimum cytotoxic pharmacophore of pancratistatin comprises the transfused B/C ring system containing the 2,3,4-triol unit in the C-ring. The results of their study further indicated that the phenanthridone skeleton in natural Amaryllidaceae alkaloids may be a significant common element for selectivity against cancer cells [62]. McNulty et al. [65] have previously demonstrated that a 2,3,4-triol-functionalized ring-C is required as the minimum pharmacophoric element for potent anticancer activity of pancratistatin.
We have recently reported that narciclasine shows potent in vitro cytotoxic activity against six different human cancer cell lines. In contrast, normal human lung fibroblasts appear to be markedly less sensitive to the molecule with a mean cytotoxic IC50 value (the concentration that reduces by 50% the number of viable cells after 3 days of treatment) of 7.5 µM compared to 0.03 µM for the six human cancer cell lines [66]. The cytotoxic activity of narciclasine was similar in the six cancer cell lines investigated, a feature consistent with NCI data that revealed a mean IC50 value of 0.046 µM for the compound across a panel of 60 cancer cell lines, including doxorubicin-resistant cancer cells [46]. Additionally, based on comparative evaluations assessing morphologic changes, after internucleosomal DNA fragmentation or the externalization of phosphatidyl serine in normal fibroblasts and in human cancer cells, the proapoptotic effects of narciclasine at concentrations up to 1 µM were confined to cancer cells [66]. These results collectively emphasize the marked selectivity of narciclasine for cancer cells, as also demonstrated by Griffin et al. [62] for pancratistatin. We have shown that the narciclasine-induced apoptosis-mediated cytotoxic effects in human cancer cells not evident in normal fibroblasts is paralleled by the triggering of the activation of the initiator caspases of the death receptor pathway (caspase-8 and -10), at least in human MCF-7 breast and PC-3 prostate carcinoma cells. This is a feature not observed in normal human fibroblasts [66]. Indeed, formation of the Fas and DR4 death-inducing signaling complex was clearly evidenced in MCF-7 and PC-3 cancer cells [66]. Additionally, in these two cancer cell lines, caspase-8 was found to interact with the Fas and DR4 receptors on narciclasine treatment. However, after this initial step, narciclasine-induced downstream apoptotic pathways in MCF-7 cells diverged from those found in PC-3 cells, where caspase-8 directly activated effector caspases such as caspase-3 in the absence of any further release of mitochondrial proapoptotic effectors [66]. In contrast, in MCF-7 cells, the apoptotic process was found to require an amplification step that is mitochondria-dependent with Bid processing, release of cytochrome c and caspase-9 activation [66].
We have postulated that the high selectivity of narciclasine for cancer cells might be linked at least in part to this activation of the death receptor pathway. Normal human fibroblasts appear approximately 250-fold less sensitive to the cytotoxic effects of narciclasine, which does not induce apoptosis in these normal fibroblasts probably due to the absence of death receptor pathway activation [67–69].Moreover, it has been suggested that Amaryllidaceae alkaloids, such as narciclasine, have a modulating activity on inflammatory reactions by inhibiting tumor necrosis factor-alpha production in stimulated macrophages [70]. Inflammation has been implicated in the development of many human epithelial cancers, including those of the stomach, lung, colon, and prostate, and tumor necrosis factor-alpha is a potent pleiotropic, proinflammatory cytokine produced by many cells in response to injury and inflammation [71].
Conclusions
Since the discovery of narciclasine, the first member of isocarbostyril class of alkaloids in 1967, the search for new natural compounds from the Amaryllidaceae plant family has resulted in the isolation of other isocarbostyrils such as lycoricidine and pancratistatin.
To date, for the synthesis of novel isocarbostyril derivatives for the potential discovery and development of new anticancer drug candidates, the best starting material for these products is narciclasine, which can be isolated from Narcissus species with a good yield. The total synthesis of these products is still very complex and requires too many steps to be developed commercially for the synthesis and anticancer testing of novel isocarbostyrils.
The biologic activities of isocarbostyril alkaloids include excellent in vitro and in vivo cytotoxicity against many tumor cell lines and high selectivity for cancer cells versus normal cells. This has encouraged several research groups to identify new derivatives or analogs of these isocarbostyril alkaloids for preclinical development. It is to be hoped that continuing interest in structure modulation of these isocarbostyril alkaloids will provide novel promising anticancer agents.
References
- 1.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60(1):52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66(7):1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 3.Hartwell JL. Plants used against cancer. A survey. Lloydia. 1967;30(4):379–436. [PubMed] [Google Scholar]
- 4.Weniger B, Italiano L, Beck JP, Bastida J, Bergoñon S, Codina C, Lobstein A, Anton R. Cytotoxic activity of Amaryllidaceae alkaloids. Planta Med. 1995;61(1):77–79. doi: 10.1055/s-2006-958007. [DOI] [PubMed] [Google Scholar]
- 5.Ibn-Ahmed S, Khaldi M, Chrétien F, Chapleur Y. A short route to enantiomerically pure benzophenanthridone skeleton: synthesis of lactone analogues of narciclasine and lycoricidine. J Org Chem. 2004;69(20):6722–6731. doi: 10.1021/jo049153l. [DOI] [PubMed] [Google Scholar]
- 6.Ghosal S, Singh S, Kumar Y, Srivastava S. Isocarbostyril alkaloids from Haemanthus kalbreyeri. Phytochemistry. 1989;28(2):611–613. [Google Scholar]
- 7.Pettit GR, Pettit GR, III, Backhaus RA, Boyd MR, Meerow AW. Antineoplastic agents: 256. Cell growth inhibitory isocarbostyrils from Hymenocallis. J Nat Prod. 1993;56(10):1682–1687. doi: 10.1021/np50100a004. [DOI] [PubMed] [Google Scholar]
- 8.Ceriotti G. Narciclasine: an antimitotic substance from Narcissus bulbs. Nature. 1967;213(5076):595–596. doi: 10.1038/213595a0. [DOI] [PubMed] [Google Scholar]
- 9.Piozzi F, Fuganti C, Mondelli R, Ceriotti G. Narciclasine and narciprimine. Tetrahedron. 1968;24(11):1119–1131. [Google Scholar]
- 10.Mondon A, Krohn K. Revision der Narciprimin-Struktur. Chem Ber. 1970;103(9):2729–2743. [Google Scholar]
- 11.Piozzi F, Marino L, Fuganti C, Di Martino A. Occurrence of nonbasic metabolites in Amaryllidaceae. Phytochemistry. 1969;8(9):1745–1748. [Google Scholar]
- 12.Okamoto T, Torii Y, Isogai Y. Lycoricidinol and lycoricidine, new plant-growth regulators in the bulbs of Lycoris radiata herb. Chem Pharm Bull. 1968;16(9):1860–1864. doi: 10.1248/cpb.16.1860. [DOI] [PubMed] [Google Scholar]
- 13.Numata A, Takemura T, Ohbayashi H, Katsuno T, Yamamoto K, Sato K, Kobayashi S. Antifeedants for the larvae of the yellow butterfly, Eurema hecabe mandarina, in Lycoris radiata. Chem Pharm Bull. 1983;31(6):2146–2149. [Google Scholar]
- 14.Pettit GR, Pettit GR, III, Backhaus RA, Boettner FE. Antineoplastic agents: 294. Variations in the formation of pancratistatin and related isocarbostyrils in Hymenocallis littoralis. J Nat Prod. 1995;58(1):37–43. doi: 10.1021/np50115a004. [DOI] [PubMed] [Google Scholar]
- 15.Pettit GR, Pettit GR, III, Groszek G, Backhaus RA, Doubek DL, Barr R, Meerow AW. Antineoplastic agents: 301. An investigation of the Amaryllidaceae genus Hymenocallis. J Nat Prod. 1995;58(5):756–759. doi: 10.1021/np50119a017. [DOI] [PubMed] [Google Scholar]
- 16.Pettit GR, Meng Y, Herald DL, Knight JC, Day JF. Antineoplastic agents: 553. The Texas grasshopper Brachystola magna. J Nat Prod. 2005;68(8):1256–1258. doi: 10.1021/np0402367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuganti C, Staunton J, Battersby AR. The biosynthesis of narciclasine. J Chem Soc D: Chem Commun. 1971;19:1154–1155. [Google Scholar]
- 18.Fuganti C, Mazza M. The absolute configuration of narciclasine: a biosynthetic approach. J Chem Soc Chem Commun. 1972;4:239. [Google Scholar]
- 19.Ghosal S, Lochan R, Ashutosh Kumar Y, Srivastava RS. Alkaloids of Haemanthus kalbreyeri. Phytochemistry. 1985;24(8):1825–1828. [Google Scholar]
- 20.Ghosal S, Singh SK, Srivastava RS. Alkaloids of Zephyranthes flava. Phytochemistry. 1986;25(8):1975–1978. [Google Scholar]
- 21.Abou-Donia AH, De Giulio A, Evidente A, Gaber M, Habib AA, Lanzetta R, Seif El Din AA. Narciclasine-4-O-β-d-glucopyranoside, a glucosyloxy amidic phenanthridone derivative from Pancratium maritimum. Phytochemistry. 1991;30(10):3445–3448. [Google Scholar]
- 22.Pettit GR, Cragg GM, Singh SB, Duke JA, Doubek DL. Antineoplastic agents: 162. Zephyranthes candida. J Nat Prod. 1990;53(1):176–178. doi: 10.1021/np50067a026. [DOI] [PubMed] [Google Scholar]
- 23.Pettit GR, Melody N. Antineoplastic agents: 527. Synthesis of 7-deoxynarcistatin, 7-deoxy-trans-dihydronarcistatin, and trans-dihydronarcistatin 1. J Nat Prod. 2005;68(2):207–211. doi: 10.1021/np0304518. [DOI] [PubMed] [Google Scholar]
- 24.Pettit GR, Gaddamidi V, Herald DL, Singh SB, Cragg GM, Schmidt JM, Boettner FE, Williams M, Sagawa Y. Antineoplastic agents: 120. Pancratium littorale. J Nat Prod. 1986;49(6):995–1002. doi: 10.1021/np50048a005. [DOI] [PubMed] [Google Scholar]
- 25.Pettit GR, Eastham SA, Melody N, Orr B, Herald DL, McGregor J, Knight JC, Doubek DL, Pettit GR, III, Garner LC, et al. Isolation and structural modification of 7-deoxynarciclasine and 7-deoxy-trans-dihydronarciclasine. J Nat Prod. 2006;69(1):7–13. doi: 10.1021/np058068l. [DOI] [PubMed] [Google Scholar]
- 26.Pettit GR, Gaddamidi V, Cragg GM, Herald DL, Sagawa Y. Isolation and structure of pancratistatin. J Chem Soc Chem Commun. 1984;24:1693–1694. [Google Scholar]
- 27.Gabrielsen B, Monath TP, Huggins JW, Kefauver DF, Pettit GR, Groszek G, Hollingshead M, Kirsi JJ, Shannon WM, Schubert EM, et al. Antiviral (RNA) activity of selected Amaryllidaceae isoquinoline constituents and synthesis of related substances. J Nat Prod. 1992;55(11):1569–1581. doi: 10.1021/np50089a003. [DOI] [PubMed] [Google Scholar]
- 28.Ouarzane-Amara M, Franetich JF, Mazier D, Pettit GR, Meijer L, Doerig C, Desportes-Livage I. In vitro activities of two antimitotic compounds, pancratistatin and 7-deoxynarciclasine, against Encephalitozoon intestinalis, a microsporidium causing infections in humans. Antimicrob Agents Chemother. 2001;45(12):3409–3415. doi: 10.1128/AAC.45.12.3409-3415.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosal S, Datta K, Singh SK, Kumar Y. Telastaside a stress-related alkaloid-conjugate from Polytela gloriosa, an insect feeding on Amaryllidaceae. J. Chem Res. 1990;10:334–335. [Google Scholar]
- 30.Kojima K, Mutsuga M, Inoue M, Ogihara Y. Two alkaloids from Zephyranthes carinata. Phytochemistry. 1998;48(7):1199–1202. [Google Scholar]
- 31.Rinner U, Hudlicky T. Synthesis of Amaryllidaceae constituents—an update. Synlett. 2005;3:365–387. [Google Scholar]
- 32.Hudlicky T, Rinner U, Gonzales D, Akgun H, Schilling S, Siengalewicz P, Martinot TA, Pettit GR. Total synthesis and biological evaluation of Amaryllidaceae alkaloids: narciclasine, ent-7-deoxypancratistatin, regioisomer of 7-deoxypancratistatin, 10b-epi-deoxypancratistatin, and truncated derivatives. J Org Chem. 2002;67(25):8726–8743. doi: 10.1021/jo020129m. [DOI] [PubMed] [Google Scholar]
- 33.Gibson DT, Hensley M, Yoshioka H, Mabry TJ. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 34.Hudlicky T, Olivo HF. A short synthesis of (+)-lycoricidine. J Am Chem Soc. 1992;114(24):9694–9696. [Google Scholar]
- 35.Tian X, Hudlicky T, Konigsberger K. First total synthesis of (+)-pancratistatin: an usual set of problems. J Am Chem Soc. 1995;117(12):3643–3644. [Google Scholar]
- 36.Doyle TJ, Hendrix M, VanDerveer D, Javanmard S, Haseltine JA. Convergent synthesis of (+)-pancratistatin based on intramolecular electrophilic aromatic substitution. Tetrahedron. 1997;53(32):11153–11170. [Google Scholar]
- 37.Trost BM, Pulley SR. Asymmetric synthesis of (+)-pancratistatin. J Am Chem Soc. 1995;117(40):10143–10144. [Google Scholar]
- 38.Rigby JH, Maharoof USM, Mateo ME. Studies on the narciclasine alkaloids: total synthesis of (+)-narciclasine and (+)-pancratistatin. J Am Chem Soc. 2000;122(28):6624–6628. [Google Scholar]
- 39.Magnus P, Sebhat IK. Application of the β-azidonation reaction to the synthesis of the antitumor alkaloid (+)-pancratistatin. Tetrahedron. 1998;54(51):15509–15524. [Google Scholar]
- 40.Ko H, Kim E, Park JE, Kim D, Kim S. Total synthesis of pancratistatin relying on the [3,3]-sigmatropic rearrangement. J Org Chem. 2004;69(1):112–121. doi: 10.1021/jo035371n. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Wu A, Zhou P. A concise synthesis of (+)-pancratistatin using pinitol as a chiral building block. Tetrahedron Lett. 2006;47(22):3707–3710. [Google Scholar]
- 42.Pettit GR, Melody N, Herald DL. Antineoplastic agents: 450. Synthesis of (+)-pancratistatin from (+)-narciclasine as relay. J Org Chem. 2001;66(8):2583–2587. doi: 10.1021/jo000710n. [DOI] [PubMed] [Google Scholar]
- 43.Pettit GR, Melody N. Synthesis of pancratistatin. WIPO patent WO0250023. 2002 [June 27] [Google Scholar]
- 44.Mondon A, Krohn K. Zur kenntris des narciclasins. Chem Ber. 1975;108(2):445–463. [Google Scholar]
- 45.Pettit GR, Melody N, Herald DL. Antineoplastic agents: 511. Direct phosphorylation of phenpanstatin and pancratistatin. J Nat Prod. 2004;67(3):322–327. doi: 10.1021/np030299+. [DOI] [PubMed] [Google Scholar]
- 46.NCI database. Available at: http://dtp.nci.nih.gov/
- 47.Rinner U, Hudlicky T, Gordon H, Pettit GR. A β-carboline-1-one mimic of the anticancer Amaryllidaceae constituent pancratistatin: synthesis and biological evaluation. Angew Chem Int Ed Engl. 2004;43(40):5342–5346. doi: 10.1002/anie.200460218. [DOI] [PubMed] [Google Scholar]
- 48.Pettit GR, Melody N, Simpson M, Thompson M, Herald DL, Knight JC. Antineoplastic agents: 500. Narcistatin. J Nat Prod. 2003;66(1):92–96. doi: 10.1021/np020225i. [DOI] [PubMed] [Google Scholar]
- 49.Pettit GR, Orr B, Ducki S. Synthesis of pancratistatin prodrugs. WIPO patent WO02085848. 2002 [October 31] [PubMed] [Google Scholar]
- 50.Pettit GR, Melody N. Pancratistatin cyclic phosphate prodrugs an phenpanstatin cyclic phosphate prodrugs. US Patent US2006128668. 2006 [June 15] [Google Scholar]
- 51.Pettit GR, Melody N. Narcistatin prodrugs. WIPO patent WO2004052298. 2004 [June 24] [Google Scholar]
- 52.Pettit GR, Melody N. Synthesis of sodium narcistatin and related compounds. WIPO patent WO2006076726. 2006 Jul 20; [Google Scholar]
- 53.McNulty J, Mao J, Gibe R, Mo R, Wolf S, Pettit GR, Herald DL, Boyd MR. Studies directed towards the refinement of the pancratistatin cytotoxic pharmacophore. Bioorg Med Chem Lett. 2001;11(2):169–172. doi: 10.1016/s0960-894x(00)00614-4. [DOI] [PubMed] [Google Scholar]
- 54.Rinner U, Hillebrenner HL, Adams DR, Hudlicky T, Pettit GR. Synthesis and biological activity of some structural modifications of pancratistatin. Bioorg Med Chem Lett. 2004;14(11):2911–2915. doi: 10.1016/j.bmcl.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Pettit GR, Melody N, O'Sullivan M, Thompson MA, Herald DL, Coates B. Synthesis of 10b-R-hydroxy-pancratistatin via narciclasine. J Chem Soc Chem Commun. 1994;23:2725–2726. [Google Scholar]
- 56.Bi Y, Guo J, Zhang L, Wong Y. Changes in some enzymes of microbodies and plastid development in excised radish cotyledons: effect of narciclasine. J Plant Physiol. 2003;160(9):1041–1049. doi: 10.1078/0176-1617-00911. [DOI] [PubMed] [Google Scholar]
- 57.Van Doorn WG, Sinz A, Tomassen MM. Daffodil flowers delay senescence in cut Iris flowers. Phytochemistry. 2004;65(5):571–577. doi: 10.1016/j.phytochem.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Jimenez A, Santos A, Alonso G, Vazquez D. Inhibitors of protein synthesis in eukaryotic cells. Comparative effects of some Amaryllidaceae alkaloids. Biochim Biophys Acta. 1976;425(3):342–348. doi: 10.1016/0005-2787(76)90261-6. [DOI] [PubMed] [Google Scholar]
- 59.Baez A, Vazquez D. Binding of [3H]narciclasine to eukaryotic ribosomes. A study on a structure-activity relationship. Biochim Biophys Acta. 1978;518(1):95–103. doi: 10.1016/0005-2787(78)90119-3. [DOI] [PubMed] [Google Scholar]
- 60.Dall'acqua F, Rodighiero C. Investigations on the mechanism of action of narciclasine. Farmaco. 1977;32(1):67–75. [PubMed] [Google Scholar]
- 61.McLachlan A, Kekre N, McNulty J, Pandey S. Pancratistatin: a natural anti-cancer compound that targets mitochondria specifically in cancer cells to induce apoptosis. Apoptosis. 2005;10(3):619–630. doi: 10.1007/s10495-005-1896-x. [DOI] [PubMed] [Google Scholar]
- 62.Griffin C, Sharda N, Sood D, Nair J, McNulty J, Pandey S. Selective cytotoxicity of pancratistatin-related natural Amaryllidaceae alkaloids: evaluation of the activity of two new compounds. Cancer Cell Int. 2007;7:10. doi: 10.1186/1475-2867-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pandey S, Kekre N, Naderi J, McNulty J. Induction of apoptotic cell death specifically in rat and human cancer cells by pancratistatin. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(3):279–295. doi: 10.1081/bio-200066621. [DOI] [PubMed] [Google Scholar]
- 64.Kekre N, Griffin C, McNulty J, Pandey S. Pancratistatin causes early activation of caspase-3 and the flipping of phosphatidyl serine followed by rapid apoptosis specifically in human lymphoma cells. Cancer Chemother Pharmacol. 2005;56(1):29–38. doi: 10.1007/s00280-004-0941-8. [DOI] [PubMed] [Google Scholar]
- 65.McNulty J, Larichev V, Pandey S. A synthesis of 3-deoxydihydrolycoricidine: refinement of a structurally minimum pancratistatin pharmacophore. Bioorg Med Chem Lett. 2005;15(23):5315–5318. doi: 10.1016/j.bmcl.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 66.Dumont P, Ingrassia L, Rouzeau S, Ribaucour F, Thomas S, Roland I, Darro F, Lefranc F, Kiss R. The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts. Neoplasia. 2007;9(9):766–776. doi: 10.1593/neo.07535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang XD, Nguyen T, Thomas WD, Sanders JE, Hersey P. Mechanisms of resistance of normal cells to TRAIL induced apoptosis vary between different cell types. FEBS Lett. 2000;482(3):193–199. doi: 10.1016/s0014-5793(00)02042-1. [DOI] [PubMed] [Google Scholar]
- 68.Zhu H, Ling W, Hu B, Su Y, Qiu S, Xiao W, Qi Y. Adenovirus E1A reverses the resistance of normal primary human lung fibroblast cells to TRAIL through DR5 upregulation and caspase 8-dependent pathway. Cancer Biol Ther. 2006;5(2):180–188. doi: 10.4161/cbt.5.2.2332. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka T, Yoshimi M, Maeyama T, Hagimoto N, Kuwano K, Hara N. Resistance to Fas-mediated apoptosis in human lung fibroblast. Eur Respir J. 2002;20(5):359–368. doi: 10.1183/09031936.02.00252602. [DOI] [PubMed] [Google Scholar]
- 70.Yui S, Mikami M, Mikami Y, Sashida Y, Yamazaki M. Inhibition effect of Amaryllidaceae alkaloids, lycorine and lycoricidinol on macrophage TNF-alpha production. Yakugaku Zasshi. 2001;121(2):167–171. doi: 10.1248/yakushi.121.167. [DOI] [PubMed] [Google Scholar]
- 71.Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66(23):11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]