Abstract
It has recently been shown that fixed-dose-rate (gemcitabine) infusion may be superior to bolus gemcitabine in the treatment of metastatic pancreas cancer. We wished to compare the radiosensitizing effects of fixed-dose-rate gemcitabine infusion to standard bolus injection. We measured weight loss and mouse intestinal crypt survival to determine equally toxic concentrations of gemcitabine administered through a 3-hour fixed-dose-rate infusion versus bolus injection in combination with fractionated radiation. To measure the effect of fixed-dose-rate gemcitabine infusion or bolus injection on radiosensitization, we treated mice bearing Panc-1 xenografts with equally toxic concentrations of gemcitabine (100 mg/kg fixed-dose-rate infusion or 500 mg/kg bolus injection) and fractionated radiation and monitored tumor growth. We found that 100 mg/kg gemcitabine through fixed-dose-rate infusion produced the same weight loss and intestinal crypt toxicity as the 500 mg/kg bolus injection. In nude mice bearing Panc-1 xenografts, fixed-dose-rate gemcitabine infusion produced greater radiosensitization than bolus injection with tumor doubling times of 44 ± 5 versus 29 ± 3 days, respectively (*P < .05). Fixed-dose-rate gemcitabine infusion produced enhanced radiosensitization without additional normal tissue toxicity compared to bolus gemcitabine injection. These data support an ongoing clinical trial using fixed-dose-rate gemcitabine infusion combined with conformal radiation in the treatment of locally advanced pancreatic cancer.
Introduction
Chemoradiation is the standard treatment for locally advanced pancreas cancer [1]. For the last 12 years, we have investigated gemcitabine combined with radiation for the treatment of pancreatic cancer both in the laboratory and the clinic. Our philosophy in the design of clinical trials for pancreatic cancer has been to give the maximal safe dose of radiation using optimal conformal techniques, along with the best systemic therapeutic agent, which is also a radiation sensitizer. This approach initially led us to combine gemcitabine with radiation, because gemcitabine has been found to be more effective than 5-fluorouracil for patients with locally advanced or metastatic pancreas cancer [2], and gemcitabine is a potent radiation sensitizer in pancreatic cancer cells [3]. Our first clinical studies established the maximum safe dose of radiation (36 Gy in 2.4-Gy fractions) that could be used with full-dose (1000 mg/m2) gemcitabine and suggested that gemcitabine-radiation therapy was at least equal to, if not better than, 5-fluorouracil-radiation therapy [4,5]. In a subsequent trial, we added cisplatin, based on the clinical finding that cisplatin-gemcitabine appeared to be superior to gemcitabine alone for metastatic disease [6] and our preclinical study of cisplatin-gemcitabine showing synergistic toxicity and equal radiosensitization compared to gemcitabine [7]. Our clinical result suggested that the full systemic dose of cisplatin and gemcitabine could be achieved in combination with conformal tumor (as well as involved regional lymph nodes) radiation, and that this combination may provide additional benefit beyond that offered by gemcitabine and radiation [8].
While several studies were being conducted in an effort to increase the effectiveness of gemcitabine-radiation therapy in pancreas cancer [4,9,10], by means of additional drugs [8,11–13], investigations were also being conducted to determine whether fixed-dose-rate infusion of gemcitabine might be more effective than the standard delivery of gemcitabine through bolus infusion. The concept underlying this approach was based on pharmacokinetic studies which showed that fixed-dose-rate gemcitabine infusion results in increased accumulation of 2′,2′-difluorodeoxycytidine 5′-triphosphate (dFdCTP), an active gemcitabine metabolite, in comparison to conventional gemcitabine delivery [14,15]. This is related to the inhibition of deoxycytidine kinase, the rate-limiting enzyme in gemcitabine metabolism, which becomes saturated in response to the standard 30-minute gemcitabine infusion. In contrast, fixed-dose-rate infusion of gemcitabine over 1 to 3 hours has been shown to prolong the steady state plasma concentrations of gemcitabine within a range (10–20 µM) that does not oversaturate deoxycytidine kinase and thus produces increased dFdCTP accumulation. A randomized phase II trial conducted by Tempero et al. [16] showed that fixed-dose-rate infusion compared to standard infusion of gemcitabine in patients with pancreatic cancer resulted in two-fold higher levels of dFdCTP in circulating mononuclear cells. Furthermore, their study suggested that fixed-dose-rate infusion might offer some clinical benefit over the standard bolus infusion of gemcitabine.
Because evidence suggested that fixed-dose-rate infusion of gemcitabine may be a better systemic therapy than standard bolus gemcitabine, we reasoned that it would be of interest to explore this as a radiosensitizing approach. We hypothesized that fixed-dose-rate infusion of gemcitabine would be a more effective radiation sensitizer than bolus injection of gemcitabine based on the higher levels of dFdCTP, and presumably 2′,2′-difluorodeoxycytidine 5′-diphosphate (dFdCDP), associated with fixed-dose-rate infusion of gemcitabine. Therefore, we carried out a study to assess whether fixed-dose-rate gemcitabine would produce at least as good, if not a better therapeutic index as standard bolus gemcitabine. We first determined comparable doses of gemcitabine for fixed-dose-rate infusion versus bolus injection using loss of body weight as an end point. Because one of the main dose-limiting toxicities for the combination of gemcitabine and radiation in the treatment of pancreas cancer is gastrointestinal toxicity related to the loss of intestinal crypts, we assessed the toxicity of fixed-dose-rate infusion and bolus injection of gemcitabine in combination with radiation by measuring the survival of intestinal crypts. We then compared the effects of fixed-dose-rate infusion versus bolus injection gemcitabine on the growth of subcutaneous pancreatic cancer tumor xenografts.
Materials and Methods
Cell Lines and Drug Administration
The human pancreatic adenocarcinoma cell line Panc-1 was obtained from American Type Culture Collection (Manassas, VA) and was maintained in RPMI 1640 medium with 10% cosmic calf serum (Hyclone, Logan, UT) and antibiotics at 37°C in 5% CO2. Gemcitabine (a gift from Eli Lilly, Indianapolis, IN) was dissolved in isotonic saline and administered once as an intraperitoneal bolus injection (500–1000 mg/kg) or as an intraperitoneal fixed-dose-rate infusion (100–750 mg/kg at 0.012–0.09 mg/min over a period of 3 hours) in animal studies. For the infusion, mice were anesthetized with a combination of ketamine and xylazine and were placed on a warming blanket to minimize heat loss. Core body temperatures were monitored with a thermocouple probe (Physitemp, Clifton, NJ) to ensure no significant change in temperature occurred during the infusion period.
Flow Cytometry
Cells were trypsinized, washed, and resuspended in PBS, fixed by dropwise addition of ice-cold 70% ethanol, and stored at 4°C until the day of analysis. Cells were then washed with PBS and suspended in PBS containing 18 µg/ml propidium iodide and 40 µg/ml ribonuclease A. Samples were analyzed on an Elite flow cytometer (Coulter Electronics, Hialeah, FL).
Clonogenic Cell Survival Assay
Clonogenic assays were performed using standard techniques as described previously [17]. Radiation survival data from drug-treated cells were corrected for plating efficiency using a nonirradiated plate treated with drug under the same conditions. Cell survival curves were fitted using the linear-quadratic equation, and the mean inactivation dose was calculated according to the method of Fertil et al. [18]. The cell survival enhancement ratio was calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose after drug exposure. A value significantly greater than 1 indicates radiosensitization.
Irradiation
Irradiations were carried out using an X-ray unit (Pantak Therapax DXT 300 Model; Pantak, East Haven, CT) at a dose rate of approximately 3 Gy/min. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration. To assess toxicity (intestinal crypt assay), animals were given 5 or 15 Gy of whole-body radiation delivered in five equal fractions beginning 24 hours post-gemcitabine treatment. Animals were maintained in restraints and positioned such that the secondary collimator encompassed the entire dorsal surface of the animal. To measure efficacy, two 6-Gy doses of radiation at 6 and 24 hours after gemcitabine delivery were given directly to the tumor. Animals were placed in a restraint that permitted them to remain conscious during the procedure and positioned such that the apex of each flank tumor was at the center of a 2.4-cm aperture in the secondary collimator and irradiated with the rest of the mouse being shielded from radiation.
Weight Loss Studies
Non-tumor-bearing C3H female mice were given gemcitabine by fixed-dose-rate infusion or bolus injection. Body weight was recorded for 1 week after gemcitabine treatment. The maximally tolerated doses for both conditions were defined as those that produced approximately a 10% weight loss.
Intestinal Crypt Assay
The microcolony assay introduced by Withers and Elkind [19] was used to determine the survival of crypt epithelial cells in the jejunum of mice exposed to whole-body irradiation (WBI). Non-tumor-bearing C3H female mice were given gemcitabine, and then on the following day, were exposed to fractionated daily WBI. Wholebody irradiation was delivered as 1- or 3-Gy daily fractions in the morning for 5 consecutive days for a total dose of 5 or 15 Gy, respectively. Mice were euthanized 66 hours after the fifth radiation dose. A 2-cm section of the jejunum was fixed in neutral-buffered formalin and was prepared for histologic examination. The regenerating crypts in the jejunal cross-section were microscopically counted at a magnification of x100 on hematoxylin and eosin-stained sections cut at a thickness of 4 µm. Data are presented as the average surviving fractions of the initial number of crypts per circumference in control for each group against the dose of WBI from three to four animals per treatment group with four sections of the jejunum from each animal and three circumferences per section.
Tumor Growth Studies
Panc-1 cells (5 x 106) were transplanted subcutaneously into the flank of Athymic Nude-Foxn1nu mice (Harlan, Indianapolis, IN). Treatment was started once a tumor reached 100 mm3. Animals were given gemcitabine and then two 6-Gy doses of radiation at 6 and 24 hours after gemcitabine treatment. Body weight and tumor size were measured three times per week. Tumor volume (TV) was calculated according to the equation for a prolate spheroid: , where a and b are the longer and shorter dimensions of the tumor, respectively. Data are expressed as the ratio of tumor volume at varying times after treatment compared to the first day of treatment (day 0). Measurements were made until day 60 or until the tumor volume increased by approximately a factor of 8, at which point the animals were sacrificed to avoid potential discomfort. Animals were handled according to the established procedures of the University of Michigan Laboratory Animals Maintenance Manual.
Statistics
Statistically significant differences were determined using the Student's t test. Statistical significance was defined at P < .05.
Results
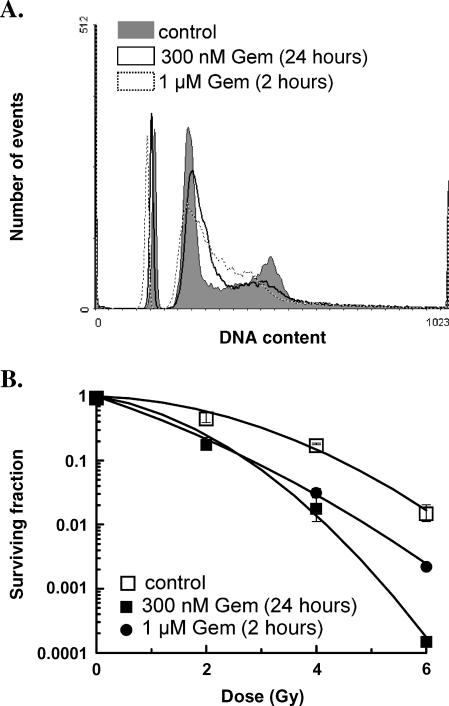
To confirm that a relatively brief exposure to gemcitabine could produce radiosensitization in pancreatic cancer cells, we began by determining if a 2-hour gemcitabine exposure could produce S-phase redistribution and radiosensitization. We confirmed in Panc-1 cells that equicytotoxic (approximately 50% survival) doses of gemcitabine administered as either a 24-hour incubation, which we have used in the past [3], or a 2-hour incubation followed by a 24-hour culture without drug resulted in redistribution of cells into early S-phase (Figure 1A). Furthermore, we found that either short or long gemcitabine exposure resulted in radiosensitization, with mean radiation enhancement ratios (± SEM) of 1.5 ± 0.2 and 1.8 ± 0.2 (n = 3), respectively (Figure 1B). Therefore, we conclude that the radiosensitization produced by a brief exposure to a high concentration of gemcitabine compared to a longer exposure to a lower concentration of gemcitabine is similar.
Figure 1.
The effect of short versus long gemcitabine exposure on cell cycle distribution and radiosensitization. Panc-1 cells were treated with the equicytotoxic concentrations of gemcitabine (Gem) for 2 or 24 hours (1 µM and 300 µM, respectively). (A) Cells were processed for flow cytometry 24 hours after the start of gemcitabine exposure. Human lymphocytes were included as an internal standard. (B) Alternatively, cells were treated with 0- to 6-Gy irradiation 24 hours from the start of gemcitabine exposure. After radiation exposure, cells were processed for clonogenic survival as described in Materials and Methods. Data are from a single experiment representative of n = 3 experiments. The mean radiation enhancement ratios ± SEM (n = 3) for short or long gemcitabine exposures were 1.5 ± 0.2 and 1.8 ± 0.2, respectively. These values were significantly different from irradiated, non-drug-treated controls (P < .05) but not significantly different from each other.
Our goal in the animal studies was to examine the relative efficacy of fixed-dose-rate infusion gemcitabine compared to bolus injection gemcitabine under the maximally tolerated dose for both conditions. In response to a single dose of gemcitabine through fixed-dose-rate or bolus injection, animals experienced weight loss during the first week after treatment but then recovered by 8 to 10 days after gemcitabine exposure. We found that a dose of 100 mg/kg given as a fixed-dose-rate infusion or 500 mg/kg given as a bolus injection produced similar toxicity, which established the doses to be used for the subsequent studies of intestinal toxicity and tumor growth delay (Figure 2).
Figure 2.
The effect of gemcitabine dose on the weight of tumor-free mice after fixed-dose-rate infusion or bolus injection gemcitabine. Mice were untreated or treated with 100- to 750-mg/kg gemcitabine by fixed-dose-rate infusion or with 500 to 1000 mg/kg by bolus injection. Total body weight was measured 10 days after treatment. Data are expressed as the percentage of the initial starting weight and are shown as the mean of three to five animals per treatment group.
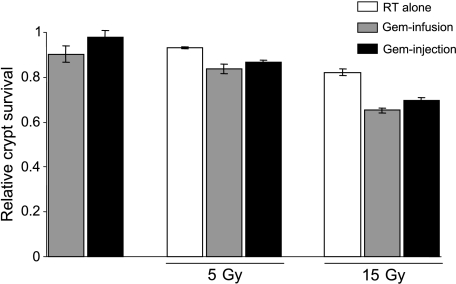
To compare the intestinal tissue toxicity of the two types of drug administration with radiation therapy, we used the jejunal crypt assay. This seemed appropriate in the context of optimizing treatment of pancreatic cancer, because the small intestine is in the radiation treatment portal. Mice were treated with a single dose of gemcitabine by fixed-dose-rate infusion or bolus injection, and on the next day, radiation treatments were initiated. We used either 5 or 15 Gy given in five fractions, which alone produced a decrease in the number of crypts. Both drug regimens (100 mg/kg infusion or 500 mg/kg bolus injection), when combined with radiation, resulted in greater damage to the jejunum than did radiation alone but were not significantly different from each other (Figure 3). In the absence of radiation, gemcitabine alone through fixed-dose-rate infusion or bolus injection produced similar effects on crypt survival. These findings, combined with the measured weight loss, suggested that we had established approximately equally toxic and maximally tolerable conditions.
Figure 3.
The effect of fixed-dose-rate versus bolus gemcitabine infusion with radiation on jejunal crypt survival. Mice were treated with 100-mg/kg gemcitabine through fixed-dose-rate infusion or with 500-mg/kg gemcitabine through bolus injection. Twenty-four hours after gemcitabine treatment, mice were given fractionated daily whole-body radiation (RT) (5 and 15 Gy at 1 or 3 Gy/day for 5 days, respectively) and were euthanized 66 hours after the fifth radiation dose. A section of the jejunum was fixed, and regenerating crypts were counted. Data are presented as the mean surviving fraction of crypts per circumference relative to the untreated control animals for each group (n = 3–4 animals per treatment group; four sections per animal were averaged to obtain a single value for each animal). There were statistically significant differences among the untreated, the radiation-treated, and the radiation- plus gemcitabine-treated groups (n = 3–4; P < .05); however, there was no significant difference between gemcitabine fixed-dose-rate infusion and bolus injection either in the absence or in the presence of 5- or 15-Gy radiation.
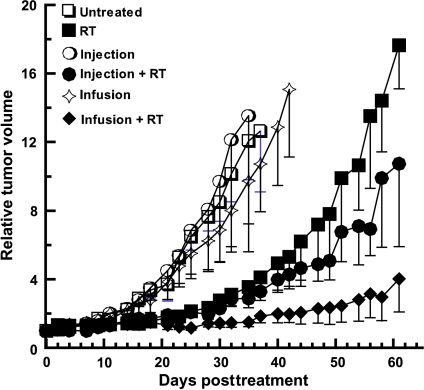
We then compared the effects of these two equitoxic treatments on the growth of Panc-1 tumors after radiation treatment. To maximize the radiosensitizing potential of gemcitabine, radiation was given (in two 6-Gy fractions) within the first 24 hours after gemcitabine treatment. We found that neither method of gemcitabine administration alone resulted in a significant slowing of tumor growth. As expected, radiation alone delayed tumor growth. The use of bolus gemcitabine caused modest radiosensitization, but radiosensitization produced by fixed-dose-rate infusion gemcitabine was greater (Figure 4). This is evidenced by a significantly slower tumor doubling time of 44 ± 5 days in animals treated with radiation and gemcitabine by fixed-dose-rate infusion gemcitabine compared to the tumor doubling time of 29 ± 3 days in animals treated with radiation and a bolus gemcitabine injection. None of these treatment conditions were accompanied by significant weight loss, suggesting minimal normal tissue toxicity (Table 1).
Figure 4.
The effect of fixed-dose-rate versus bolus injection gemcitabine on pancreatic tumor xenograft growth. Nude mice bearing Panc-1 tumor xenografts were treated with 100-mg/kg gemcitabine through fixed-dose-rate infusion or with 500-mg/kg gemcitabine through bolus injection. Mice were then exposed to 12-Gy radiation given in two fractions at 6 and 24 hours post-gemcitabine treatment. Tumor growth was measured for 60 days after treatment. Data are presented as the mean tumor volume relative to the starting volume on the first day of treatment (day 0) (untreated: n = 3, RT: n = 4, bolus: n = 4, bolus + RT: n = 5, infusion: n = 4, infusion + RT: n = 5). There was a statistically significant difference in the time to tumor doubling between radiation plus gemcitabine fixed-dose-rate infusion (44 ± 5 days) versus radiation plus gemcitabine bolus injection (29 ± 3 days) (*P < .05).
Table 1.
Relative* Weight After Therapy.
| Treatment | Day 2 | Day 7 |
| Untreated | 1.02 ± 0.01 | 1.05 ± 0.02 |
| RT | 0.99 ± 0.02 | 1.03 ± 0.01 |
| Injection | 1.03 ± 0.06 | 1.07 ± 0.06 |
| Injection + RT | 0.98 ± 0.03 | 1.12 ± 0.06 |
| Infusion | 1.00 ± 0.01 | 0.99 ± 0.01 |
| Infusion + RT | 1.01 ± 0.02 | 0.96 ± 0.02 |
Weights are relative to the first day of treatment (day 0).
Discussion
In this study, we have found that the combination of radiation and fixed-dose-rate gemcitabine produced a better therapeutic index than radiation plus standard bolus gemcitabine. As the main goal of this work was to design an improved treatment for pancreatic cancer, we used a human pancreas tumor xenograft model to assess efficacy. Because the major dose-limiting toxicity for the combination of gemcitabine and radiation in the treatment of pancreas cancer is gastrointestinal (specifically, duodenal), we chose weight loss and an assessment of intestinal crypts to measure toxicity. These findings, when combined with previous clinical data suggesting that fixed-dose-rate gemcitabine might be superior to bolus gemcitabine as systemic treatment, support the concept of fixed-dose-rate infusion of gemcitabine as a reasonable option to explore in combination with radiation in the treatment of locally unresectable pancreatic cancer.
There have been a number of attempts to improve the chemotherapeutic effects of bolus gemcitabine alone for patients with metastatic pancreatic cancer. A recent phase III clinical trial in pancreas cancer compared the efficacy of the combination of cisplatin and gemcitabine to gemcitabine alone [20]. This study demonstrated a significant improvement in progression-free survival and a trend toward improvement in the overall survival for the combination of cisplatin and gemcitabine compared to gemcitabine alone. We conducted a phase I/II trial using a time to event continual reassessment model to determine that essentially the same dose of cisplatin and gemcitabine as was used for metastatic disease could be given with conformal radiation for patients with locally advanced disease and found a median survival of 13 months [8], which was promising compared to historical controls [4].
Oxaliplatin has also been added to gemcitabine in an effort to improve the treatment of advanced pancreatic cancer [21]. A randomized phase III trial showed improved progression-free survival and a nonstatistically significant trend toward improved overall survival in patients treated with gemcitabine-oxaliplatin versus gemcitabine alone. Based on clinical data and our preclinical data that oxaliplatin and gemcitabine show synergistic toxicity, and that radiosensitization is maintained, we have initiated a phase I/II trial of oxaliplatin dose escalation in combination with gemcitabine-radiation. This combination was well tolerated but whether it will be superior to gemcitabine-radiation therapy is not yet determined. In the latter part of this study, an attempt will be made to replace bolus gemcitabine with fixed-dose-rate gemcitabine, which is supported by the results of this current study.
The radiosensitizing and cytotoxic properties of gemcitabine are mediated by two distinct metabolites: dFdCDP and dFdCTP, respectively. The diphosphorylated metabolite of gemcitabine, dFdCDP, is a potent inhibitor of ribonucleotide reductase, resulting in reduced synthesis of deoxynucleoside triphosphates, primarily deoxyadenosine triphosphate. Previous studies have shown a correlation between the doses of gemcitabine required to achieve maximal radiosensitization and those that produced nearly complete deoxyadenosine triphosphate pool depletion, whereas intracellular concentrations of dFdCTP were found not to correlate with radiosensitization [22,23]. Instead, dFdCTP has been shown to mediate the cytotoxic properties of gemcitabine through misincorporation of 2′,2′-difluorodeoxycytidine 5′-monophosphate into the DNA [24,25]. Together, these studies suggest that dFdCDP mediates radiosensitization, whereas dFdCTP is responsible for cytotoxicity. dFdCTP levels have been shown to accumulate at two-fold higher levels in the peripheralmononuclear blood cells of patients that received fixed-dose-rate infusion gemcitabine versus bolus gemcitabine [16]. Because dFdCDP is the precursor for dFdCTP and the levels of these two metabolites have been shown to correlate [26], it is likely that fixed-dose-rate infusion also results in elevated dFdCDP levels. Moreover, it will be important in future studies to determine whether fixed-dose-rate infusion gemcitabine also results in increased tumor concentrations of dFdCTP or dFdCDP.
This study has some limitations. We chose to conduct the tumor growth delay studies with a different radiation scheme than that used in the toxicity studies. Whereas it would have been ideal to use the same radiation schemes, this was not technically feasible. Mouse intestine is much more sensitive to radiation than implanted tumor. We chose two 6-Gy fractions for the tumor growth delay experiments because we wanted 1) to produce maximal radiosensitization, which has been shown to occur in the first 24 hours after gemcitabine [22] and 2) to avoid possible differences in gemcitabine metabolism for the two different delivery methods that might have occurred over a longer course of several radiation fractions and confounded the assessment of radiosensitization. This dose (6 Gy x 2) of whole-body radiation would result in a high mortality rate. Conversely, if we had administered the tolerable whole-body dose (1 Gy or 3 Gy x 5) to the tumor, we would not have expected to see substantial tumor responses. We feel that the conclusion of this experiment does not depend on the particular dose of radiation, but on the fact that we assessed the effects of the same doses of radiation for the two gemcitabine delivery methods on tumor growth delay under conditions in which the drug-radiation combination was equally toxic.
In the present study, we used an intraperitoneal bolus injection of gemcitabine. However, the standard administration of gemcitabine in the clinic is through a 30-minute intravenous infusion. Because we were interested in the basic principle of a short versus a longer gemcitabine administration, we elected to compare an intraperitoneal bolus injection to a 3-hour fixed-dose-rate intraperitoneal infusion. Although an intraperitoneal injection results in a slower uptake than an intravenous bolus injection, it is likely that the pharmacokinetics of a bolus intraperitoneal injection are different from a 30-minute intravenous infusion and that the pharmacokinetics in mice are different from humans. Therefore, whereas this study demonstrates that fixed-dose-rate infusion produces greater tumor radiosensitization than bolus injection in mice, it should be followed up with clinical trials to test this hypothesis in patients.
This study suggests that fixed-dose-rate gemcitabine should be explored to try to improve the outcome of treatment for pancreatic cancer and supports the incorporation of fixed-dose-rate gemcitabine into future clinical trials. This is consistent with our approach to clinical trial design with the goal of improving systemic therapy while maintaining optimal local therapy. However, since a recent analysis suggests that, with long-term follow-up, a substantial minority of patients will still have local failure [27], improvement in local control should also be sought. This could be achieved through improved radiosensitization, such as might be achieved by fixed-dose-rate gemcitabine or potentially by improved technical approaches such as the use of intensity-modulated radiotherapy (IMRT). It is plausible that IMRT might play a role in this context, as bowel toxicity is the major toxicity, and might permit even more intense dose systemic therapy with full-dose radiation therapy. However, we feel that these efforts using IMRT should only be done in the context of a clinical trial. We have recently initiated such a study.
Footnotes
This work was supported by the National Institutes of Health grant RO1 CA78554 and the University of Michigan Cancer Center support grant 5 P30 CA46592. No conflicts of interest exist.
References
- 1.Abrams RA. Adjuvant therapy for pancreatic adenocarcinoma: what have we learned since 1985? Int J Radiat Oncol Biol Phys. 2003;56:3–9. doi: 10.1016/s0360-3016(03)00451-6. [DOI] [PubMed] [Google Scholar]
- 2.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 4.McGinn CJ, Zalupski MM, Shureiqi I, Robertson JM, Eckhauser FE, Smith DC, Brown D, Hejna G, Strawderman M, Normolle D, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Frytak S, Hahn RG, O'Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Philip PA, Zalupski MM, Vaitkevicius VK, Arlauskas P, Chaplen R, Heilbrun LK, Adsay V, Weaver D, Shields AF. Phase II study of gemcitabine and cisplatin in the treatment of patients with advanced pancreatic carcinoma. Cancer. 2001;92:569–577. doi: 10.1002/1097-0142(20010801)92:3<569::aid-cncr1356>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Symon Z, Davis M, McGinn CJ, Zalupski MM, Lawrence TS. Concurrent chemoradiotherapy with gemcitabine and cisplatin for pancreatic cancer: from the laboratory to the clinic. Int J Radiat Oncol Biol Phys. 2002;53:140–145. doi: 10.1016/s0360-3016(01)02790-0. [DOI] [PubMed] [Google Scholar]
- 8.Muler JH, McGinn CJ, Normolle D, Lawrence T, Brown D, Hejna G, Zalupski MM. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22:238–243. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 9.Blackstock AW, Bernard SA, Richards F, Eagle KS, Case LD, Poole ME, Savage PD, Tepper JE. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999;17:2208–2212. doi: 10.1200/JCO.1999.17.7.2208. [DOI] [PubMed] [Google Scholar]
- 10.Crane CH, Antolak JA, Rosen II, Forster KM, Evans DB, Janjan NA, Charnsangavej C, Pisters PW, Lenzi R, Papagikos MA, et al. Phase I study of concomitant gemcitabine and IMRT for patients with unresectable adenocarcinoma of the pancreatic head. Int J Gastrointest Cancer. 2001;30:123–132. doi: 10.1385/IJGC:30:3:123. [DOI] [PubMed] [Google Scholar]
- 11.Safran H, Dipetrillo T, Iannitti D, Quirk D, Akerman P, Cruff D, Cioffi W, Shah S, Ramdin N, Rich T. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a phase I trial. Int J Radiat Oncol Biol Phys. 2002;54:137–141. doi: 10.1016/s0360-3016(02)02902-4. [DOI] [PubMed] [Google Scholar]
- 12.Blackstock AW, Melin SA, Butler JM, Patton S, Pineau B, Albertson D, Howerton R, Levine E. Irinotecan/gemcitabine followed by twice-weekly gemcitabine/radiation in locally advanced pancreatic cancer. Oncology (Williston Park) 2002;16:25–28. [PubMed] [Google Scholar]
- 13.Kachnic LA, Shaw JE, Manning MA, Lauve AD, Neifeld JP. Gemcitabine following radiotherapy with concurrent 5-fluorouracil for nonmetastatic adenocarcinoma of the pancreas. Int J Cancer. 2001;96:132–139. doi: 10.1002/ijc.1008. [DOI] [PubMed] [Google Scholar]
- 14.Cattel L, Airoldi M, Delprino L, Passera R, Milla P, Pedani F. Pharmacokinetic evaluation of gemcitabine and 2′,2′-difluorodeoxycytidine-5′-triphosphate after prolonged infusion in patients affected by different solid tumors. Ann Oncol. 2006;17(suppl 5):v142–v147. doi: 10.1093/annonc/mdj970. [DOI] [PubMed] [Google Scholar]
- 15.Grunewald R, Abbruzzese JL, Tarassoff P, Plunkett W. Saturation of 2′,2′-difluorodeoxycytidine 5′-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol. 1991;27:258–262. doi: 10.1007/BF00685109. [DOI] [PubMed] [Google Scholar]
- 16.Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, Abbruzzese J. Randomized phase II comparison of doseintense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence TS. Ouabain sensitizes tumor cells but not normal cells to radiation. Int J Radiat Oncol Biol Phys. 1988;15:953–958. doi: 10.1016/0360-3016(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 18.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 19.Withers HR, Elkind MM. Radiosensitivity and fractionation response of crypt cells of mouse jejunum. Radiat Res. 1969;38:598–613. [PubMed] [Google Scholar]
- 20.Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 21.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, Zaniboni A, Ducreux M, Aitini E, Taieb J, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Shewach DS, Hahn TM, Chang E, Hertel LW, Lawrence TS. Metabolism of 2′,2′-difluoro-2′-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res. 1994;54:3218–3223. [PubMed] [Google Scholar]
- 23.Shewach DS, Lawrence TS. Radiosensitization of human tumor cells by gemcitabine in vitro. Semin Oncol. 1995;22:68–71. [PubMed] [Google Scholar]
- 24.Ostruszka LJ, Shewach DS. The role of DNA synthesis inhibition in the cytotoxicity of 2′,2′-difluoro-2′-deoxycytidine. Cancer Chemother Pharmacol. 2003;52:325–332. doi: 10.1007/s00280-003-0661-5. [DOI] [PubMed] [Google Scholar]
- 25.Huang P, Plunkett W. Fludarabine- and gemcitabine-induced apoptosis: incorporation of analogs into DNA is a critical event. Cancer Chemother Pharmacol. 1995;36:181–188. doi: 10.1007/BF00685844. [DOI] [PubMed] [Google Scholar]
- 26.Losa R, Sierra MI, Gion MO, Esteban E, Buesa JM. Simultaneous determination of gemcitabine di- and triphosphate in human blood mononuclear and cancer cells by RP-HPLC and UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840:44–49. doi: 10.1016/j.jchromb.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 27.Murphy JD, Adusumilli S, Griffith KA, Ray ME, Zalupski MM, Lawrence TS, Ben-Josef E. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]