Abstract
This study aimed to profile the methylation statuses of CDH1/E-cadherin and five CpG island methylator phenotype (CIMP)-associated genes (p16, hMLH1, MINT1, MINT2, and MINT31) in gastric specimens of 47 Dalian long-term residents with and 31 without gastric cancers (GCs). CIMP patterns were classified as CIMP-H with over three methylated genes, CIMP-L with one to two methylated genes, and CIMP-N without methylation. Of 47 GC cases, 24 (51.1%) were CIMP-H, 18 (38.3%) were CIMP-L, and 5 (10.6%) were CIMP-N, whereas 5 of 21 (23.8%) premalignant lesions were CIMP-H and 15 (71.4%) were CIMP-L. CIMP-L was found in 75% (12/16) of GC-adjacent mucosa and in 38.7% (12/31) of mucosa from GC-free patients. CDH1 methylation occurred in 48.9% (23/47) of cancer, in 23.8% (5/21) of premalignant, and in 25% (4/16) of noncancerous tissues and was correlated with patients' age (P = .01), lymph node metastasis, and CIMP severity (P = .000–.028). Our results demonstrated that the frequencies of CIMP-H in Dalian GCs, CIMP-L, and p16 methylation in GC-adjacent tissues and in GC-free mucosa were much higher than those reported previously, indicating the elevated methylation pressure in this GC high-risk region. The close correlation between CDH1 methylation and CIMP severity suggests the necessity of their combination in GC prevention and earlier diagnosis.
Introduction
Gastric cancer (GC) is one of the commonest malignancies in the world and the major cause of cancer-related deaths in China [1]. There are several GC at-risk regions in China including Dalian, a coastal and mountainous area located at the far south of Northeast China. Although great effort has been paid in early prevention, diagnosis, and treatments, GC remains the first killer malignancy among Dalian residents due to later diagnosis and lack of timely surveillance measures. Multiple regional oncogenic factors have been proposed during the past decades and diet habits are one of them [2]. Epidemiologic studies demonstrated that people in this area were accustomed to eating toasted or salted seafood and meat and preparing preserved vegetables for the long winter season. These foods are rich in gastrocarcinogens such as heterocyclic amines, polycyclic aromatic hydrocarbons, and the well-known DNA methylators such as nitrite and benzo(a)pyrene [3,4]. Apparently, a long-term consumption of those foods may enhance the risk of genetic and epigenetic alterations [5]. The high GC incidence and the traditional diet habits in this region provide unique resource for investigating molecular aspect of gastrocarcinogenesis.
DNA hypermethylation is the main machinery of epigenetic modulation of gene expression [6], especially for those induced by chemical reagents [7,8]. DNA methylation happens in definite sequences because most of the methylation occurs on the cytosine of CpG site, where the 5′-CG-3′ dinucleotide sequences appear infrequently in the genome [9]. Because the 5′-promoter region of most genes are rich in CpG sequences [10], their methylation statuses determine the activity of gene transcription [6,11]. A body of evidence demonstrated that alterations of DNA methylation are closely related with cancer formation, particularly with those raised from the organs exposed directly to environmental carcinogens [7]. Because methylated genes rarely come alone but in group [12], Toyota et al. [13,14] introduced the concept of CpG island methylator phenotype (CIMP) through the study of aberrant methylation in colorectal and GCs, in which five to seven methylator-sensitive genes were included for evaluating the methylation statuses in cancers and for correlating the CIMP pattern( s) with tumor risk and prevention [15,16].
So far, no comprehensive study has been performed on Dalian GCs concerning CIMP profiling and its potential relationship with the high GC incidence. Cyclooxygenase-2 (COX-2) has been shown to play oncogenic roles during stepwise gastrocarcinogenesis, and its expression is correlated with Helicobacter pylori infection, nuclear factor-kappa B activation, and Wnt signaling [17–19]. However, despite the existence of those COX-2 stimulators, COX-2 expression remains infrequent in Dalian GCs due to hypermethylation of the COX-2 promoter [20]. These data exclude the feasibility of COX-2-targeting therapy for the prevention of Dalian GCs and, meanwhile, highlight a hypermethylation-related gastrocarcinogenic option in this GC at-risk region. We therefore hypothesized that, in addition to COX-2 hypermethylation, global methylation status in gastric epithelial cells might also be altered, which would be favorable for GC initiation and progression. This hypothesis was tested in this study by profiling the patterns of CIMP and CDH1 methylation in GC mass, GC surrounding tissue, and grossly normal-looking epithelium of the surgical specimens as well as the endoscopic gastric mucosa of GC-free patients.
Materials and Methods
Sample Collection and Treatment
Forty-seven surgical specimens from 34 male (mean age 61.6 ± 9.2 years) and 13 female (mean age 61.7 ± 8.8 years) GC patients were selected from the Frozen Gastric Tissue Bank of the Cancer Institute, Dalian Medical University (DMU), Dalian, PR China. Gastric mucosa were obtained from 31 cancer-free patients and were provided after the test of H. pylori infection and/or frozen section-based pathologic examination. All of the patients were long-term Dalian residents who never received preoperative chemotherapy. The tissue samples were selected from the GC mass, GC surrounding tissue, and grossly normal-looking epithelium of the surgical specimens. They were trimmed to suitable sizes on ice, snap-frozen immediately in liquid nitrogen, and stored at -85°C until use. All tissue preparations were completed within 20 minutes of tissue removal. After obtaining patients, consent, fresh gastric biopsies were collected from either the Operation Rooms at DMU First Affiliated Hospital or the Gastroendoscopic Department of DMU Second Affiliated Hospital. The frozen tissue blocks were sectioned in 5-µm thickness, fixed in cold acetone for 20 minutes, and subjected to hematoxylin and eosin (H&E) staining for pathologic reexamination and tissue composition evaluation. According to pathologic findings, the gastric tissues were classified into well-differentiated intestinal-type gastric cancer (i-GC), poorly differentiated diffuse-type gastric cancer (d-GC), premalignant lesions including intestinal metaplasia and atrophic gastritis, and normal-looking noncancerous mucosa. Meanwhile, a piece of placenta tissue was obtained after getting the permission from a labored donor.
Genomic DNA Preparation and Sodium Bisulfite Treatment
Sample preparations were conducted according to the findings of pathologic examination. When a tissue block showed uniform composition, it was sectioned directly for DNA isolation. When multiple tissue components were found in the same tissue block, the border of the target histologic region was marked precisely for cell type-defined sample preparations with the manual dissection method established in our laboratory [21,22]. In total, 16 to 24 pieces of 3-µm target frozen fragments were collected after sectioning and were placed immediately in a 1.5-ml Eppendorf tube containing 300 µl of TE buffer (pH 8.0, 1% sodium dodecyl sulfate), digested with 5 µl of proteinase K (10 mg/ml), and subjected to conventional DNA extraction using phenol/chloroform extraction and ethanol precipitation.
For chemical DNA modification, 5 µl of sample DNA (0.4 µg/µl) was mixed with 40 µl of pure water, heated at 97°C for 6 minutes, and interacted with 5 µl of 2 M NaOH at 37°C for 10 minutes, mixed with 30 µl of freshly prepared 10 mM hydroquinone (H9003; Sigma, St. Louis, MO) and 520 µl of 3 M sodium bisulfate (S9000; Sigma), and then incubated at 50°C in darkness for 16 to 20 hours. The chemical-modulated sample DNA was subjected to desalt purification using the Wizard DNA Clean-Up System (A7280; Promega, Madison, WI). The purified DNA was dissolved with 50 µl of pure water, mixed with 5.5 µl of 3 M NaOH at 37°C for 5 minutes, precipitated in ethanol, and dissolved in 30 µl of pure water for use. A placenta DNA sample was incubated with Sss1 methyltransferase (New England Biolabs Inc., Beverly, MA) at 37°C for 4 hours followed by bisulfite treatment.
Methylation-Specific Polymerase Chain Reaction (PCR)
The sequences of methylation-specific PCR (MSP) primers and the conditions of PCR reaction for individual genes are listed in Table 1 [23]. PCR amplifications were conducted using TaKaRa PCR amplification Kit (TaKaRa Biotechnology Inc. Dalian, China) by the following procedures: 30 µl of reaction solution was prepared, which contains 3 µl of 10x buffer (Mg2+ Plus), 4 µl of 2.5 mM deoxyribonucleotide triphosphate, 0.5 µl of respective 20 µM upstream and downstream primers, 1 U Taq polymerase (5 U/µl), and 5 µl of DNA template. The reaction condition was as follows: 94°C for 5 minutes; 94°C for 1 minute; 53 to 65°C for 1 minute and 72°C for 1 minute for 35 to 40 cycles; and 72°C for 5 minutes. The PCR products were separated in 6% polyacrylamide gel and were then observed and photographed under UV illumination (BioSpectrumAC BioImaging Systems; Ultra-Violet Products Inc., Upland, CA). Distilled pure water was used as negative control [23] and the placenta DNA was treated with Sss1 methyltransferase was used as positive control for methylated allele in each of MSP reactions [24].
Table 1.
Primer Sequences and MSP Reaction Conditions for Individual Genes.
| Gene | Primer Sequence | Annealing Temperature (°C) |
| p16 | Methylated | 65 |
| Sense 5′-TTATTAGAGGGTGGGGCGGATCGC-3′ | ||
| Antisense 5′-GACCCCGAACCGCGACCGTAA-3′ | ||
| Unmethylated | 60 | |
| Sense 5′-TTATTAGAGGGTGGGGTGGATTGT-3′ | ||
| Antisense 5′-CAACCCCAAACCACAACCATAA-3′ | ||
| MINT1 | Methylated | 55 |
| Sense 5′-AATTTTTTTATATATATTTTCGAAGC-3′ | ||
| Antisense 5′-AAAAACCTCAACCCCGCG-3′ | ||
| Unmethylated | 55 | |
| Sense 5′-AATTTTTTTATATATATTTTTGAAGTGT-3′ | ||
| Antisense 5′-AACAAAAAACCTCAACCCCACA-3′ | ||
| MINT2 | Methylated | 60 |
| Sense 5′-TTGTTAAAGTGTTGAGTTCGTC-3′ | ||
| Antisense 5′-AATAACGACGATTCCGTACG-3′ | ||
| Unmethylated | 60 | |
| Sense 5′-GATTTTGTTAAAGTGTTGAGTTTGTT-3′ | ||
| Antisense 5′-CAAAATAATAACAACAATTCCATACA-3′ | ||
| MINT31 | Methylated | 60 |
| Sense 5′-TGTTGGGGAAGTGTTTTTCGGC-3′ | ||
| Antisense 5′-CGAAAACGAAACGCCGCG-3′ | ||
| Unmethylated | 64 | |
| Sense 5′-TAGATGTTGGGGAAGTGTTTTTTGGT-3′ | ||
| Antisense 5′-TAAATACCCAAAAACAAAACACCACA-3′ | ||
| hMLH1 | Methylated | 60 |
| Sense 5′-GATAGCGATTTTTAACGC-3′ | ||
| Antisense 5′-TCTATAAATTACTAAATCTCTTCG-3′ | ||
| Unmethylated | 60 | |
| Sense 5′-AGAGTGGATAGTGATTTTTAATGT-3′ | ||
| Antisense 5′-ACTCTATAAATTACTAAATCTCTTCA-3′ | ||
| CDH1 | Methylated | 57 |
| Sense 5′-TTAGGTTAGAGGGTTATCGCGT-3′ | ||
| Antisense 5′-TAACTAAAAATTCACCTACCGAC-3′ | ||
| Unmethylated | 53 | |
| Sense 5′-TAATTTTAGGTTAGAGGGTTATTGT-3′ | ||
| Antisense 5′-CACAACCAATCAACAACACA-3′ |
Criteria of CIMP Classification
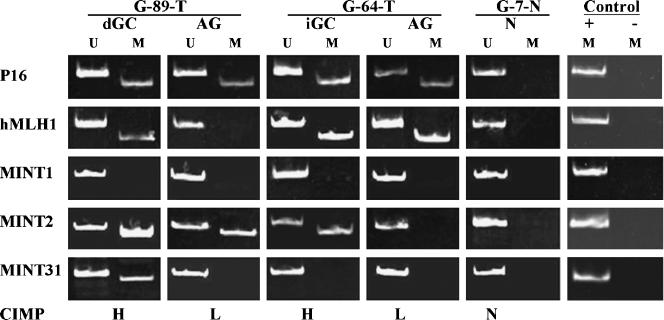
In this study, p16, hMLH1, MINT1, MINT2, and MINT31 were chosen and the hot loci of CpG island methylation in their promoter regions were examined for CIMP identification. According to the suggestion of Jean-Pierre Issa,s Laboratory [25], the grades of methylation were classified into three types: CIMP-high (CIMP-H) when three to five genes were found with methylation; CIMP-low (CIMP-L) when one or two genes were methylated; and CIMP-none (CIMP-N) if none of the five genes was methylated. As indicated in Figure 1, DNA samples isolated from the GC tissues of G-89-T and G-64-T showed CIMP-H pattern, whereas their premalignant counterparts revealed CIMP-L, and the noncancerous mucosa showed CIMP-N. Meanwhile, the CpG island methylation in the CDH1 promoter region was checked using the PCR primers and reaction condition described elsewhere [26] and the results were compared with CIMP patterns.
Figure 1.
Illustration of CIMP-H, CIMP-L, and CIMP-N and their distribution in different gastric tissues. U and M indicate the PCR products amplified with the primers for unmethylated and methylated sequences, respectively. d-GC, diffuse gastric cancer; i-GC, intestinal gastric cancer; AG, chronic atrophic gastritis. Normal placenta DNA treated with Sss1 methyltransferase was used as positive control (+) and distilled water without template DNA as negative control (-) for methylated loci.
Collection and Analyses of CIMP-Related Data
The related clinical data were obtained from the clinical records achieved in the Case Muniment Room of DMU Affiliated Hospitals. Patients, gender, age, Lauren's classification, the degree of tumor differentiation, clinical stage/tumor, node, and metastasis staging, and H. pylori infection were recorded. Mann-Whitney test was used to analyze the distribution of three CIMP patterns as well as CDH1 methylation in GC, premalignant, and noncancerous groups, and their relation with patients, gender, age, GC subtypes, tumor, node, and metastasis staging, and H. pylori infection. Spearman relative analysis was employed to evaluate the relation of the extent of CIMP and CDH1 methylation.
Results
Differential Methylation Patterns of Five CIMP-Related Genes during Stepwise Gastrocarcinogenesis
The same parameters (p16, hMLH1, MINT1, MINT2, and MINT31) proposed by other investigators were employed here as CIMP-related genes [23]. As shown in Table 2, MSP analysis performed on the tissues obtained from gastrectomy specimens revealed that the incidences of p16 methylation were 18.8% (3/16) in noncancerous, 57.1% (12/21) in premalignant, and 63.8% (30/47) in GC tissues, respectively. The detection rates of GCs and premalignant lesions were significantly different with their noncancerous counterpart (P = .002 and P = .020). The rates of hMLH1 methylation were 25% (4/16), 47.6% (10/21), and 53.2% (25/47) in noncancerous, premalignant, and GC groups, respectively. In the case of MINTs, the methylation rates of MINT1 were 25% (4/16) in noncancerous, 42.9% (9/21) in premalignant, and 44.7 (21/47) in GC groups; MINT2, 12.5% (2/16) in noncancerous, 33.3% (7/21) in premalignant, and 40.4 (19/47) in GC groups; and MINT31, 18.8% (3/16) in noncancerous, 14.3% (3/21) premalignant, and 25.5 (12/47) GC groups. Unlike the results of p16 analysis, Mann-Whitney test revealed that no significant difference between the hMLH1, MINT1, MINT2, or MINT31 methylation rates could be found among the three histologic groups when they were compared in pair (P > .05).
Table 2.
Methylation Statuses of p16, hMLH1, MINT1, MINT2, MINT31, and CDH1 in Different Gastric Tissues.
| Locus | Methylation Frequencies | P | |||
| Endoscopic (n = 31) |
Noncancerous (n = 16) |
Premalignant (n= 21) |
GC (n = 47) | ||
| p16 | 29% (9/31) | 18.8% (3/16) | 57.1% (12/21) | 63.8% (30/47) | .008 |
| hMLH1 | 9.7% (3/31)* | 25% (4/16) | 47.6% (10/21) | 53.2% (25/47) | .151 |
| MINT1 | 6.5% (2/31)* | 25% (4/16) | 42.9% (9/21) | 44.7% (21/47) | .375 |
| MINT2 | 0% (0/31)* | 12.5% (2/16) | 33.3% (7/21) | 40.4% (19/47) | .126 |
| MINT31 | 9.7% (3/31)* | 18.8% (3/16) | 14.3% (3/21) | 25.5% (12/47) | .56 |
| CDH1 | 9.7% (3/31)* | 25% (4/16) | 23.8% (5/21) | 48.9% (23/47) | .031 |
Significant difference of methylation incidences between the noncancerous mucosa of the patients with and without gastric cancers (Mann-Whitney test, P = .000–.008).
Distinct CIMP Patterns of GC, Premalignant, and Noncancerous Groups
As summarized in Figure 2, and Table 3, the frequencies of CIMP-H were 51.1% (24/47) in GC group, 23.8% (5/21) in premalignant lesions, and 0% (0/16) in noncancerous mucosa. CIMP-L was 38.3% (18/47) in GCs, 71.4% (15/21) in premalignant, and 75% (12/16) in noncancerous tissues, respectively. CIMP-N was 10.6% (5/47) in GCs, 4.8% (1/21) in premalignant, and 25% (4/16) in noncancerous tissues. The statistical analyses revealed that the incidences of CIMP-H were significantly different (P = .001) between premalignant (23.8%, 5/21) and GC groups (51.1%, 24/47).However, no statistical difference could be established between diffuse GCs (59.3%, 16/27) and intestinal GCs (40%, 8/20) (P = .282) and between the primary GCs with (50%, 15/30) and without lymph node metastases (50%, 5/10) (P = .435).
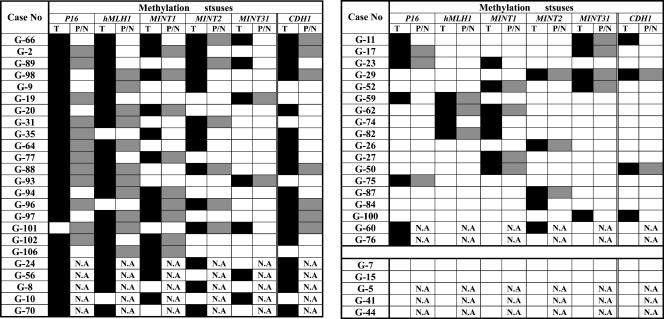
Figure 2.
Profiling of CIMPs in gastric cancers raised from Dalian, China. ■, the locus with methylation among GC tissues; □, the locus with methylation among premalignant/noncancerous gastricmucosa; and ▒, the locus without methylation. N.A., tissue sample was not available.
Table 3.
Differential CIMP and CDH1 Methylation Patterns during Stepwise Gastrocarcinogenesis and Their Correlation with Patients' Personal Parameters.
| Parameters | No. | CIMP | P |
CDH1* Methylation (%) |
P | ||
| H (%) | L (%) | N (%) | |||||
| Gender | |||||||
| Male | 34 | 16 (47.1) | 13 (38.2) | 5 (14.7) | .317 | 16 (47.1) | .108 |
| Female | 13 | 8 (61.5) | 5 (38.5) | 0 (0) | 7 (53.8) | ||
| Age (years) | |||||||
| < 50 | 12 | 4 (33.4) | 7 (58.3) | 1 (8.3) | .154 | 2 (16.7) | .010 |
| ≥ 50 | 35 | 20 (57.2) | 11 (31.4) | 4 (11.4) | 21 (60) | ||
| Histology | |||||||
| Noncancerous | 16 | 0 (0) | 12 (75) | 4 (25) | .001 | 4 (25) | .031 |
| Premalignant | 21 | 5 (23.8) | 15 (71.4) | 1 (4.8) | 5 (23.8) | ||
| Cancer | 47 | 24 (51.1) | 18 (38.3) | 5 (10.6) | 23 (48.9) | ||
| d-GC | 27 | 16 (59.3) | 8 (29.6) | 3 (11.1) | .282 | 16 (59.3) | .100 |
| i-GC | 20 | 8 (40) | 10 (50) | 2 (10) | 7 (35) | ||
| Differentiation | |||||||
| Poor | 32 | 16 (50) | 13 (40.6) | 3 (9.4) | .236 | 19 (59.4) | .335 |
| Well | 7 | 4 (57.1) | 2 (28.6) | 1 (14.3) | 2 (28.6) | ||
| Lymph node metastasis |
|||||||
| + | 30 | 15 (50) | 11 (36.7) | 4 (13.3) | .435 | 19 (63.3) | .028 |
| - | 10 | 5 (50) | 5 (50) | 0 (0) | 2 (20) | ||
| H. pylori infection | |||||||
| + | 8 | 3 (37.5) | 3 (37.5) | 2 (25) | .079 | 3 (37.5) | .112 |
| - | 5 | 5 (100) | 0 (0) | 0 (0) | 4 (80) | ||
Significant difference of CDH1 methylation incidences between CIMP-H and CIMP-L/N groups (Spearman relative analysis, P = .000).
Frequent CIMP-L in the Mucosa of GC-Free Patients
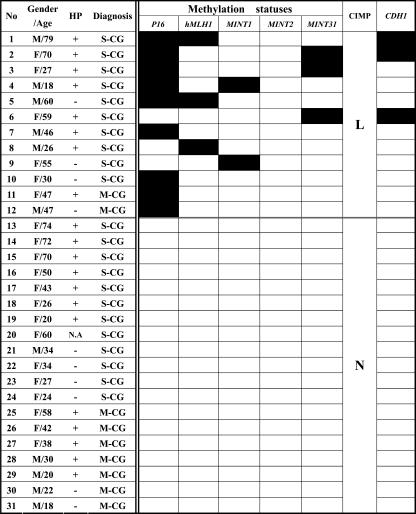
Among the 31 gastric biopsies obtained from GC-free patients, 9 were found with mild and 22 were with moderate to severe chronic gastritis (Figure 3). Furthermore, 20 were positive in H. pylori infection, 10 were negative, and 1 was without relevant record. There were nine cases (29%) with p16 methylation, three (9.7%) with hMLH1, two (6.5%) with MINT1, none with MINT2, and three (9.7%) with MINT31. None of those samples exhibited CIMP-H, whereas 12/31 (38.7%) were found with CIMP-L. Distribution of CIMP-L in mild (2/9, 22.2%) and severe gastritis (10/22, 45.5%) was different but without statistical significance (P = .367). In comparison with the data obtained from the noncancerous mucosa of GC-bearing patients, the frequencies of hMLH1, MINT1, MINT2, and MINT31 methylation were significantly lower (P = .000–.008) except that of p16 methylation (P = .323).
Figure 3.
Personal backgrounds and CIMP statuses of gastric biopsies of GC-free patients. The dark and light blocks indicate the genes with and without methylation, respectively. M-CG, mild chronic gastritis; S-CG, moderate to severe chronic gastritis; N.A., the record of H. pylori test was not available.
No Relevance of CIMP Patterns with Patients' Gender, Age, and H. pylori Infection
Analysis of CIMP patterns in male and female GC patients revealed that CIMP-H was 47.1% (16/34), CIMP-L was 38.2% (13/34), and CIMP-N was 14.7% (5/34) among male patients, and 61.5% (8/13), 38.5% (5/13), and none among females, respectively (Table 3). No significant difference of these parameters could be established between the two groups (P = .317). The 47 GC cases checked here were divided into two age groups: the younger (less than 50 years old) and the elder (over 50 years old) groups [12]. The incidences of i-GC and d-GC were 50% (6/12) and 50% (6/12) in the younger and 40% (14/35) and 60% (21/35) in the elder group, respectively, showing similar i-GC and d-GC incidences of the two groups (P = .157). Of 12 younger cases, 4 (33.4%) were found with CIMP-H, 7 (58.3%) with CIMP-L, and 1 (8.3%) with CIMP-N, whereas 20 (57.2%) of 35 elder GC patients were with CIMP-H, 11 (31.4%) with CIMP-L, and 4 (11.4%) with CIMP-N. Although CIMP-H frequency of the elder patients was higher than that of the younger patients, this difference had no statistical significance (P = .154).
Among the 47 GC cases, 8 were diagnosed as H. pylori-positive (HP+) and 5 as H. pylori-negative (HP-). Notably, all of the HP-cases showed CIMP-H in comparison with 37.5% (3/8) of CIMP-H in HP+ tissues. However, a statistical difference of CIMP-H incidences could not be established (P = .079) between the two groups due to the limited case number. To further address this issue, the test of H. pylori infection was performed on the noncancerous mucosa of 30 GC-free patients, which revealed that 20 were found with and 10 without H. pylori infection. The same frequencies of CIMP-L (40%) and CIMP-N (60%) were found in both HP+ and HP- groups.
Close Correlation of CIMP-H with CDH1 Hypermethylation
CDH1 promoter methylation was found in 48.9% (23/47) of GC samples, and 75% (18/24) of this methylation occurred in CIMP-H and 21.7% (5/23) in CIMP-L/N GC group, revealing a significant difference of CDH1 methylation rates between the two groups (P = .000; Table 3). The incidences of CDH1 methylation were apparently lower in the premalignant (23.8%; 5/21) and noncancerous tissues (25%; 4/16) of GC patients (P = .031). About 35% (7/20) of i-GC were found with methylated CDH1 in comparison with 59.3% (16/27) in d-GC (P = .100), indicating that the loss of E-cadherin due to the increased CDH1 methylation in d-GCs may lead to the diffuse phenotype. Additionally, CDH1 methylation was found in 63.3% (19/30) of primary GCs with lymph node metastases, whereas in 20% (2/10) of those without lymph node metastases (P = .028). CDH1 methylation was also correlated with the patients' age (P = .010), because its frequencies were apparently different in the younger (16.7%, 2/12) and the elder GC groups (60%, 21/35). In contrast, no statistical significance of CDH1 methylation incidences was found between male (47.1%, 16/34) and female (53.8%, 7/13) patients (P = .108). The results from the noncancerous mucosa of 31 GC-free patients revealed that 3 cases (9.7%) were found with CDH1 methylation, and all of them fell into the elder and CIMP-L groups.
Discussion
Altered gene silencing due to DNA hypermethylation is a universal epigenetic event in great majority of human malignancies, which, unlike the situation of hereditary diseases, happens to multiple genes in cancer cells and the genes affected usually vary in chemical- and disease-dependent fashions [3,4,27]. It is therefore necessary to elucidate the methylation statuses of a panel of representative genes in an individual disease. To achieve this goal, CIMP was introduced by Toyota et al. [13]. Data from gastroenterological cancers revealed that a combination of MINT1, MINT2, and MINT31 with tumor suppressor p16 and mismatch repair gene hMLH1 is suitable in evaluating the general methylation status in these sorts of tumors [14,25,28]. To make our results more comparable with the published data, we employed this evaluation system to check the unknown CIMP pattern in Dalian GCs and to see whether the CIMP pattern here was different from those identified in other places.
To have a clearer view in the correlation of DNA methylation with GC formation in this GC high-risk region, different histologic tissues of GC surgical specimens and the endoscopic gastric mucosa of GC-free patients were collected and examined. It has been believed that GC-adjacent noncancerous mucosa and chronic gastritis rarely had p16 (0–10%) and hMLH1 (0–1.7%) methylation, even in the case of GCs of high-risk regions [29,30]. In contrast, the methylation incidences of p16 and hMLH1 in the same tissue types of Dalian GC patients were 18.8% and 25%, respectively. Accordingly, the methylation rates of MINT1 (25%) and MINT2 (12.5%) in those samples were also higher than the ones (5% and 7%) reported by other investigators [28]. These results implicate the existence of certain epigenetic variations in different GC high-risk regions and the elevated methylation pressure in Dalian region presumably due to its epidemiologic features as mentioned in the Introduction section. This speculation was further supported by the data obtained from the inflammatory mucosa of 31 GC-free patients, because 29% of them were found with p16 methylation, 9.7% with hMLH1, and even GC-associated MINT1 and MINT31 methylation could be detected in the rates of 6.5% and 9.7%, respectively. Moreover, p16 and MINT1 or MINT31 methylation appeared even in the mucosa of 18-year-old male and 27-year-old female patients, and hMLH1 in a 26-year-old male. If we suppose the relatively high p16 and hMLH1 methylation incidences in GC-adjacent mucosa is somewhat influenced by the neoplastic changes and/or tumorigenic microenvironment, the findings from GC-free patients reflect the frequent preexistence of abnormally methylated genes in this GC high-risk population. Because p16 plays important roles in controlling cell outgrowth [31] and hMLH1 in repairing the nucleotide sequences damaged/altered by external and intrinsic factors [32], it is reasonable to suppose that the cells without those proteins may gain longer life span and/or more chance of additional genetic aberrations.
The CIMP data from previous GC studies revealed that the frequencies of CIMP-H were from 31% to 51% in GC tissues, whereas only 4% to 16% in GC-adjacent premalignant or normal mucosa [28,33]. It was therefore proposed that CIMP-H happened in the later stage of gastrocarcinogenesis. In this study, CIMP-H was found preferentially in Dalian GCs (51.1%) and at a higher rate among premalignant lesions (23.8%, 5/21), suggesting the accumulation of multiple loci methylations during malignant transformation. Although none of the noncancerous mucosa exhibited CIMP-H, the incidence of CIMP-L (75%) in those tissues was remarkably higher than the ones (17–38%) reported previously [28,33,34] because of the early appearance of methylation(s) of the five checked genes. Based on the low frequency of COX-2 expression due to promoter hypermethylation in Dalian GCs, we had assumed the possible high methylation background in this GC high-risk population [20]. The results of the current study provided further supporting data for this speculation and motivated us to check whether the gastric mucosa of GC-free patients showed the similar methylation tendency.
The data about CIMP patterns in different gastric lesions have been accumulated in recent years, including the ones from other GC popular regions [33,34]. However, a simultaneous analysis on gastric samples from the patients with and without GCs in a highrisk population has been rarely reported. This work was conducted using endoscopic biopsies of 31 GC-free outpatients. Similar with the situation of GC-adjacent noncancerous mucosa, a high rate (38.7%) of CIMP-L and absence of CIMP-H was found in those samples. It was noted that 2 of 12 CIMP-L cases appeared in the patients with mild gastritis and 7 in the patients are less than 50 years old. These findings thus provide additional evidence for the high methylation status of Dalian long-term residents, and further hold the notion that DNA methylation may occur at the early stage of gastrocarcinogenesis. Although the local factor(s) leading to the high methylation status remains to be figured out, early detection of methylation-sensitive genes such as p16 and exploration of reliable method to erase the methylation from those genes would have potential value in GC prevention of this region.
Generally, the genes affected by hypermethylation can be classified into two categories: cancer-associated genes as Type-C and age-related genes as Type-A [13]. The five genes used for CIMP profiling here are Type-C genes [35] that are sensitive to environmental methylators [25]. However, their responses to other GC-related factors like H. pylori infection are still in dispute. Maekita et al. [36] found high levels of aberrant DNA methylation in H. pylori-infected gastric samples, whereas Kang et al. [30] reported that this epigenetic event was unrelated with H. pylori infection. The results obtained from the gastric samples of both GC-bearing and GC-free patients revealed that the CIMP status is related neither with H. pylori infection nor with patients, age, gender, as well as GC subtypes. These data thus suggest that Type-C genes are more suitable for CIMP profiling because of their preferential methylations in cancer-associated lesions regardless of the differences of other personal factors. Additionally, our results indicate two possibilities: 1) the gastrocarcinogenic effect(s) of H. pylori may not depend on DNA methylation pathway or 2) certain powerful methylation element(s) might exist universally and cause hypermethylation of Type-C genes in Dalian gastric samples irrespective of the presence or absence of H. pylori infection. In this context, more careful evaluation will be required to address the association of HP infection and increased severity of DNA methylation in GC high-risk regions.
Downregulation of CDH1 is a typical malignant biomarker observed in a wide variety of cancers including GCs [37]. Multiple genetic factors can inhibit CDH1 expression, and hypermethylation in CDH1 promoter region is one of the main reasons [38]. Because CDH1 methylation usually appeared in elder patients, it was regarded as a kind of age-related methylation (Type-A methylation) [35]. In parallel with CIMP analysis, the status of CDH1 methylation was checked here to elucidate the potential link of CDH1 methylation to CIMP patterns and to see whether this sort of methylation occurred earlier or more frequently in a GC high-risk population. In agreement with previous reports [35,39,40], we found increased frequency of CDH1 methylation in GC samples and the gastric mucosa of GC-free patients in an age-related pattern. However, it is difficult to conclude the causality of CDH1 methylation with gastrocarcinogenesis because: 1) the methylation event of CDH1 unnecessarily occurs concurrently in GC and noncancerous tissues of the same patients and 2) CDH1 methylation is frequently found in CIMP-H GC cases. Moreover, CDH1 methylation is closely related with GC dissemination because of its presence in 63.3% (19/30) of primary GCs with lymph node metastases, but only 20% (2/10) in those without lymph node metastases. All these data suggest the cancer-promoting feature of CDH1 downregulation/silencing and the existence of other methylation element(s) in the transformed cells beyond cell aging. Therefore, we would rather regard CDH1 methylation as Mixed type (Type-M) because of its compatibility with age- and cancer-associated features. To make the results more informative and substantial, a combination of CDH1 with Type-C genes would be necessary in CIMP profiling.
In summary, our comprehensive CIMP profiling revealed a high CIMP-H incidence in GC samples and more frequent CIMP-L in premalignant and noncancerous mucosa of the patients with and without GCs, suggesting the persistent methylation pressure and increased CIMP grade in this GC at-risk region. Among the five CIMP-associated genes so far checked, p16 seems more sensitive to methylator(s) because of its overall methylation rate and the earlier onset of its methylation in stepwise gastrocarcinogenesis. CDH1 methylation was closely related with either aging or malignant phenotypes of gastric epithelial cells. Therefore, an integration of CDH1 with conventional CIMP-related genes is recommended in methylation assay. Because several options for profiling methylation patterns of GCs have been available [14,28,30,34], it would be worthwhile to compare their applicability in GC risk assessment of the Dalian region based on the current study.
Footnotes
This work was supported in part by grants from the National Natural Science Foundation of China (30370384, 30527002, and 30670946).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.You WC, Blot WJ, Chang YS, Ershow AG, Yang ZT, An Q, Henderson B, Xu GW, Fraumeni JF, Jr, Wang TG. Diet and high risk of stomach cancer in Shandong, China. Cancer Res. 1988;48:3518–3523. [PubMed] [Google Scholar]
- 3.Chen CS, Pignatelli B, Malaveille C, Bouvier G, Shuker D, Hautefeuille A, Zhang RF, Bartsch H. Levels of direct-acting mutagens, total N-nitroso compounds in nitrosated fermented fish products, consumed in a highrisk area for gastric cancer in southern China. Mutat Res. 1992;265:211–221. doi: 10.1016/0027-5107(92)90050-c. [DOI] [PubMed] [Google Scholar]
- 4.Sadikovic B, Rodenhiser DI. Benzopyrene exposure disrupts DNA methylation and growth dynamics in breast cancer cells. Toxicol Appl Pharmacol. 2006;216:458–468. doi: 10.1016/j.taap.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Slattery ML, Curtin K, Sweeney C, Levin TR, Potter J, Wolff RK, Albertsen H, Samowitz WS. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2006;120:656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- 6.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;49:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 7.Marsit CJ, Karagas MR, Schned A, Kelsey KT. Carcinogen exposure and epigenetic silencing in bladder cancer. Ann N Y Acad Sci. 2006;1076:810–821. doi: 10.1196/annals.1371.031. [DOI] [PubMed] [Google Scholar]
- 8.Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–2402. doi: 10.1093/hmg/9.16.2395. [DOI] [PubMed] [Google Scholar]
- 9.Yoder JA, Walsh CP, Bestor TH. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997;13:335–340. doi: 10.1016/s0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 10.Antequera F, Bird A. Number of CpG islands and genes in human and mouse. Proc Natl Acad Sci USA. 1993;90:11995–11999. doi: 10.1073/pnas.90.24.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bird A. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- 12.Munot K, Bell SM, Lane S, Horgan K, Hanby AM, Speirs V. Pattern of expression of genes linked to epigenetic silencing in human breast cancer. Hum Pathol. 2006;37:989–999. doi: 10.1016/j.humpath.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, Baylin SB, Issa JP. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res. 1999;59:5438–5442. [PubMed] [Google Scholar]
- 15.Marsit CJ, Houseman EA, Christensen BC, Eddy K, Bueno R, Sugarbaker DJ, Nelson HH, Karagas MR, Kelsey KT. Examination of a CpG island methylator phenotype and implications of methylation profiles in solid tumors. Cancer Res. 2006;66:10621–10629. doi: 10.1158/0008-5472.CAN-06-1687. [DOI] [PubMed] [Google Scholar]
- 16.Shen L, Ahuja N, Shen Y, Habib NA, Toyota M, Rashid A, Issa JP. DNA methylation and environmental exposures in human hepatocellular carcinoma. J Natl Cancer Inst. 2002;94:755–761. doi: 10.1093/jnci/94.10.755. [DOI] [PubMed] [Google Scholar]
- 17.Juttner S, Cramer T, Wessler S, Walduck A, Gao F, Schmitz F, Wunder C, Weber M, Fischer SM, Schmidt WE, et al. Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell Microbiol. 2003;5:821–834. doi: 10.1046/j.1462-5822.2003.00324.x. [DOI] [PubMed] [Google Scholar]
- 18.Hung JH, Su IJ, Lei HY, Wang HC, Lin WC, Chang WT, Huang W, Chang WC, Chang YS, Chen CC, et al. Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-kappaB and pp38 mitogen-activated protein kinase. J Biol Chem. 2004;279:46384–46392. doi: 10.1074/jbc.M403568200. [DOI] [PubMed] [Google Scholar]
- 19.Howe LR, Suaramaiah K, Chung WJ, Dannenberg AJ, Brown AM. Transcriptional activation of cyclooxygenase-2 in Wnt-1-transformed mouse mammary epithelial cells. Cancer Res. 1999;59:1572–1577. [PubMed] [Google Scholar]
- 20.Huang L, Zhang KL, Li H, Chen XY, Kong QY, Sun Y, Gao X, Guan HW, Liu J. Infrequent COX-2 expression due to promoter hypermethylation in gastric cancers in Dalian, China. Hum Pathol. 2006;37:1557–1567. doi: 10.1016/j.humpath.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Sun Y, Kong QY, Zhang KL, Wang XW, Chen XY, Wang Q, Liu J. Combination of nucleic acid and protein isolation with tissue array construction: using defined histologic regions in single frozen tissue blocks for multiple research purposes. Int J Mol Med. 2003;12:299–304. [PubMed] [Google Scholar]
- 22.Sun Y, Chen XY, Liu J, Cheng XX, Wang XW, Kong QY, Li H. Differential caspase-3 expression in noncancerous, premalignant and cancer tissues of stomach and its clinical implication. Cancer Detect Prev. 2006;30:168–173. doi: 10.1016/j.cdp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Park SJ, Rashid A, Lee JH, Kim SG, Hamilton SR, Wu TT. Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol. 2003;162:815–822. doi: 10.1016/S0002-9440(10)63878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY. Differential DNA methylation profiles in gynecological cancers and correlation with clinicopathological data. BMC Cancer. 2006;6:212. doi: 10.1186/1471-2407-6-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 26.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang GH, Lee S, Kim WH, Lee HW, Kim JC, Rhyu MG, Ro JY. Epstein-Barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am J Pathol. 2002;160:787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An C, Choi IS, Yao JC, Worah S, Xie K, Mansfield PF, Ajani JA, Rashid A, Hamilton SR, Wu TT. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005;11:656–663. [PubMed] [Google Scholar]
- 29.Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG. CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res. 2001;61:2847–2851. [PubMed] [Google Scholar]
- 30.Kang GH, Lee HJ, Hwang KS, Lee S, Kim JH, Kim JS. Aberrant CpG island hypermethylation of chronic gastritis, in relation to aging, gender, intestinal metaplasia, and chronic inflammation. Am J Pathol. 2003;163:1551–1556. doi: 10.1016/S0002-9440(10)63511-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drayton S, Brookes S, Rowe J, Peters G. The significance of p16INK4a in cell defenses against transformation. Cell Cycle. 2004;3:611–615. [PubMed] [Google Scholar]
- 32.Fedier A, Fink D. Mutations in DNA mismatch repair genes: implications for DNA damage signaling and drug sensitivity. Int J Oncol. 2004;24:1039–1047. [PubMed] [Google Scholar]
- 33.Lee JH, Park SJ, Abraham SC, Seo JS, Nam JH, Choi C, Juhng SW, Rashid A, Hamilton SR, Wu TT. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004;23:4646–4654. doi: 10.1038/sj.onc.1207588. [DOI] [PubMed] [Google Scholar]
- 34.Chang MS, Uozaki H, Chong JM, Ushiku T, Sakuma K, Ishikawa S, Hino R, Barua RR, Iwasaki Y, Arai K, et al. CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus. Clin Cancer Res. 2006;12:2995–3002. doi: 10.1158/1078-0432.CCR-05-1601. [DOI] [PubMed] [Google Scholar]
- 35.Choi IS, Wu TT. Epigenetic alterations in gastric carcinogenesis. Cell Res. 2005;15:247–254. doi: 10.1038/sj.cr.7290293. [DOI] [PubMed] [Google Scholar]
- 36.Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 37.Cheng XX, Wang ZC, Chen XY, Sun Y, Kong QY, Liu J, Gao X, Guan HW, Li H. Frequent loss of membranous E-cadherin in gastric cancers: a cross-talk with Wnt in determining the fate of beta-catenin. Clin Exp Metastasis. 2005;22:85–93. doi: 10.1007/s10585-005-4578-8. [DOI] [PubMed] [Google Scholar]
- 38.Graziano F, Arduini F, Ruzzo A, Mandolesi A, Bearzi I, Silva R, Muretto P, Testa E, Mari D, Magnani M, et al. Combined analysis of E-cadherin gene (CDH1) promoter hypermethylation and E-cadherin protein expression in patients with gastric cancer: implications for treatment with demethylating drugs. Ann Oncol. 2004;15:489–492. doi: 10.1093/annonc/mdh108. [DOI] [PubMed] [Google Scholar]
- 39.Kang GH, Lee S, Kim JS, Jung HY. Profile of Raberrant CpG island methylation along multistep gastric carcinogenesis. Lab Invest. 2003;83:519–526. doi: 10.1097/01.lab.0000064704.53132.65. [DOI] [PubMed] [Google Scholar]
- 40.To KF, Leung WK, Lee TL, Yu J, Tong JH, Chan MW, Ng EK, Chung SC, Sung JJ. Promoter hypermethylation of tumor-related genes in gastric intestinal metaplasia of patients with and without gastric cancer. Int J Cancer. 2002;102:623–628. doi: 10.1002/ijc.10783. [DOI] [PubMed] [Google Scholar]