Abstract
Clinical evidence suggests that gemcitabine (Gem) plus oxaliplatin (Ox) is superior to gemcitabine alone in advanced pancreatic carcinoma. The addition of radiation to gemcitabine improves response and is a standard treatment for locally advanced disease. We investigated the effect of oxaliplatin on gemcitabine-based chemoradiation by determining whether gemcitabine and oxaliplatin produced synergistic cytotoxicity using median effect analysis and radiosensitization using clonogenic survival assays. We analyzed the effects of gemcitabine and oxaliplatin on cell cycle distribution by DNA content and on radiation-induced DNA damage repair by phosphorylated H2AX (γ-H2AX). Gemcitabine and oxaliplatin produced schedule-dependent synergistic cytotoxicity in BxPC-3 and Panc-1 cells (combination indices: 0.76 ± 0.05, 0.61 ± 0.11). In BxPC-3 cells, oxaliplatin did not affect gemcitabine-mediated radiosensitization (Gem 1.99 ± 0.27; Gem + Ox 2.38 ± 0.30). In Panc-1 cells, oxaliplatin significantly enhanced gemcitabine-mediated radiosensitization (Gem 1.31 ± 0.05; Gem + Ox 2.90 ± 0.31). Radiosensitization by gemcitabine was accompanied by early S-phase arrest and induction/persistence of γ-H2AX protein, which were unaltered by oxaliplatin. Addition of oxaliplatin to gemcitabine produces radiosensitization equal to or greater than gemcitabine alone, supporting our clinical investigation of oxaliplatin with gemcitabine-radiation in pancreatic cancer aimed at improving systemic disease control while maintaining local tumor radiosensitization.
Introduction
In the past decade, gemcitabine has become the standard therapy for advanced pancreatic cancer. The combination of concurrent highly conformal radiation with gemcitabine produces a median survival of 12 months [1] that compares favorably to historical controls of gemcitabine alone with a median survival of 7 months [2]. Although local tumor control is an important issue, the majority of treatment failures is due to systemic disease progression [3]. Therefore, our approach in designing clinical trials for pancreatic cancer has emphasized on improving systemic disease control while maintaining local tumor control. This approach led us to combine gemcitabine with radiation, because gemcitabine has been found to be more effective than 5-FU for patients with locally advanced or metastatic pancreas cancer [2] and because gemcitabine is a potent radiation sensitizer in pancreatic cancer cells [4]. Our initial clinical studies established the maximum safe dose of radiation (36 Gy in 2.4-Gy fractions) that could be used with full-dose (1000 mg/m2) gemcitabine and suggested that gemcitabine radiation therapy was at least equal to if not better than 5-FU-radiation therapy [1,5].
While we were pursuing the combination of gemcitabine and radiation for locally advanced pancreatic cancer, others were combining gemcitabine with cisplatin in effort to improve therapy for metastatic pancreatic cancer. Philip et al. [6] demonstrated that cisplatin could be safely administered with full-dose gemcitabine and that it potentially could improve median survival. These studies led us to combine a gemcitabine-cisplatin-based chemotherapy regimen with radiation. Our preclinical studies showed that gemcitabine and cisplatin produced synergistic cytotoxicity without compromising radiosensitization compared to gemcitabine alone [7]. This preclinical data led us to initiate a clinical trial combining gemcitabine, cisplatin, and radiation. This study suggested that the full systemic dose of cisplatin and gemcitabine could be administered in combination with conformal tumor (as well as involved regional lymph nodes) radiation and that this combination may provide additional benefit beyond that offered by gemcitabine and radiation [8]. The patient survival in this clinical trial of 13 months seemed promising compared to historical controls for gemcitabine-radiation therapy; however, increased systemic toxicity caused by the addition of cisplatin was noted.
Therefore, we and others became interested in the platinum analogue, oxaliplatin, which appears to have equal efficacy to cisplatin but less gastrointestinal, hematological, and renal toxicity. Clinical trials combining gemcitabine plus oxaliplatin versus gemcitabine alone in metastatic or locally unresectable disease demonstrated that the combination was significantly superior (P < .05) with regard to response (27% vs 17%), progression-free survival (5.8 vs 3.7 months), and clinical benefit (38% vs 27%), although the improvement in overall survival did not reach significance (median of 9 vs 7.1 months, P = .13) [9]. Based on these data suggesting that the combination gemcitabine with cisplatin or oxaliplatin is superior to gemcitabine alone, we wished to conduct a preclinical study combining gemcitabine and oxaliplatin with radiation. We designed our study to first determine whether gemcitabine and oxaliplatin could produce synergistic cytotoxicity. We then went on to assess radiosensitization in response to gemcitabine and oxaliplatin.
Materials and Methods
Cell Lines and Drug Solutions
The human pancreatic adenocarcinoma cell lines BxPC-3 and Panc-1 were obtained from American Type Culture Collection (Manassas, VA) and maintained in either RPMI 1640 or Dulbecco's modified Eagle's medium, respectively, with 10% Cosmic Calf Serum (Hyclone, Logan, UT) and antibiotics at 37°C in 5% CO2. Gemcitabine (a gift from Eli Lilly, Indianapolis, IN) and oxaliplatin (Sigma Chemical, St. Louis, MO) were dissolved in phosphate-buffered saline (PBS) and stored at -20°C.
Clonogenic Cell Survival Assay
Clonogenic assays were performed using standard techniques as described previously [10]. Drug cytotoxicity was calculated as the ratio of surviving drug-treated cells relative to untreated controls. Radiation survival data from drug-treated cells were corrected for plating efficiency using an unirradiated plate treated with drug under the same conditions. Cell survival curves were fitted using the linear-quadratic equation and the mean inactivation dose was calculated according to the method of Fertil et al. [11]. The cell survival enhancement ratio was calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose after drug exposure. A value greater than 1 indicates significant radiosensitization.
Combined Drug Effect Analysis
To examine synergy between gemcitabine and oxaliplatin, the survival of BxPC-3 or Panc-1 cells in response to fixed ratios of variable doses of gemcitabine (0.1–3 or 1–30 µM, respectively) and/or oxaliplatin (3–60 µM) (both toxic and nontoxic) was examined using the median effect analysis as described previously [12]. Because this experimental design requires the two drugs be administered in a fixed ratio, the dose of the combination required to produce fractional survival could be divided into the component doses D1 and D2 of drugs 1 and 2, respectively. The combination index (CI) was calculated according to the equation: CI = (D1/Dx1) + (D2/Dx2) + β(D1D2) / (Dx1Dx2) where Dx1 and Dx2 are the concentrations of drugs 1 and 2, when used alone that produce a surviving fraction of x, and D1 and D2 are the individual concentrations of drugs 1 and 2 in the fixed ratio, which produces a surviving fraction of x: α = 1 or 0 depending on whether the drugs are assumed to be mutually nonexclusive or mutually exclusive, respectively. Because the sigmoidicity of the dose-effect curves for gemcitabine and oxaliplatin is different, α = 1 in this study. Synergy is indicated by CI < 1, additivity by CI = 1, and antagonism by CI > 1 [12].
Irradiation
Irradiations were carried out using an X-ray unit (Pantak Therapax DXT 300; Pantak, East Haven, CT) at a dose rate of approximately 3 Gy/min. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration.
Flow Cytometry
Cells were trypsinized, washed, and resuspended in PBS, fixed by dropwise addition of ice-cold 70% ethanol, and stored at 4°C until the day of analysis. Cells were then washed with PBS and suspended in PBS containing 18 µg/ml propidium iodide and 40 µg/ml ribonuclease A. Human lymphocytes were included as internal standards. Cells were analyzed on a FACScan flow cytometer (Becton-Dickinson, Palo Alto, CA) with WinMDI software (Version 2.8; J. Trotter, Scripps Research Institute, La Jolla, CA).
Immunoblot Analysis
Whole-cell lysates were prepared in buffer containing 10 mmol/l Tris (pH 7.4), 2% sodium dodecyl sulfate, 1x Complete Protease Inhibitor Cocktail (Roche, Mannheim, Germany), 1 mmol/l sodium fluoride, 2 mmol/l sodium orthovanadate, and 1 mmol/l sodium pyrophosphate. Protein concentration was determined with the Bicinchoninic Acid Protein Assay Reagent (Pierce, Rockford, IL). Samples were diluted in loading buffer (0.32 mol/l Tris-HCl, 10% glycerol, 2% sodium dodecyl sulfate, 0.2% bromophenol blue, and 4% 2-mercaptoethanol, pH 6.8) and resolved on 4% to 12% gradient Bis-Tris gels (Invitrogen, Carlsbad, CA). Separated proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA) and hybridized overnight at 4°C with antibodies recognizing phosphorylated H2AX (Ser139) (γ-H2AX) (Upstate Biotechnology, Lake Placid, NY) or β-actin (Sigma). Membranes were then probed with secondary antibodies, incubated with an electrochemiluminescent reagent (ECL Plus; Amersham Biosciences, Little Chalfont, Buckinghamshire, UK), and exposed to film. The ImageJ program (National Institutes of Health, Bethesda, MD) was used for the quantification of the specific protein bands on film.
Statistics
Data are expressed as the mean of at least three experiments, unless otherwise indicated. Statistically significant differences were determined using Student's t test. Statistical significance was defined at P < .05.
Results
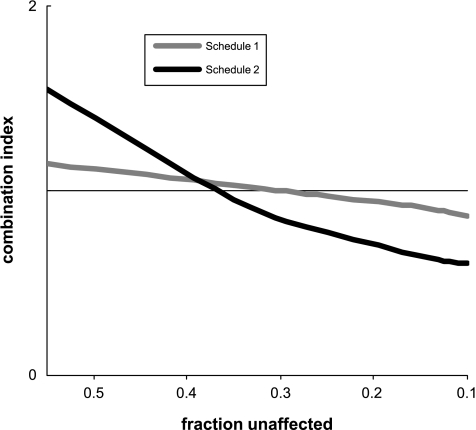
To determine how to combine gemcitabine and oxaliplatin with radiation, we first wished to assess the effects of gemcitabine and oxaliplatin on cytotoxicity.We analyzed the clonogenic survival of BxPC-3 and Panc-1 cells in response to two different schedules of gemcitabine and oxaliplatin according to the principles of median effect analysis to determine whether the selected conditions produced synergistic cell killing. For gemcitabine, cells were incubated for a 2-hour period, which should be an adequate duration for gemcitabine to deplete deoxyribonucleotide triphosphate pools [13]. For oxaliplatin, cells were incubated for 3 hours, a period sufficient for the formation of platinum-DNA adducts [14]. We investigated two different schedules: gemcitabine first followed the next day by oxaliplatin (schedule 1) or oxaliplatin first followed the next day by gemcitabine (schedule 2). Schedules were selected in order to minimize (schedule 1) or maximize (schedule 2) the potential influence of oxaliplatin on gemcitabine-mediated radiosensitization. In both of these schedules, radiation was given 24 hours after gemcitabine, conditions known to produce radiosensitization. To assess each of these schedules, we examined cell survival in response to gemcitabine and oxaliplatin. We chose a ratio of 1 part gemcitabine to 10 parts oxaliplatin based on clinical data demonstrating that standard chemotherapeutic doses of these two drugs result in substantially higher plasma concentrations of oxaliplatin relative to gemcitabine [15]. Because we were trying to model full chemotherapeutic doses of these drugs as used clinically, we focused on concentrations of gemcitabine and oxaliplatin that produced substantial cytotoxicity. The CI plot of Panc-1 cells treated under schedule 2 was < 1 at surviving fractions of < 0.4 (Figure 1) and reached statistical significance at a surviving fraction of 0.03 (Table 1), indicating synergistic cytotoxicity between gemcitabine and oxaliplatin. In BxPC-3 cells, whereas schedule 2 produced only additive cell killing, schedule 1 resulted in synergistic cell killing in response to the combination of gemcitabine and oxaliplatin at a surviving fraction of 0.03. From these data, we conclude that gemcitabine and oxaliplatin can promote synergistic cell killing in pancreatic cancer cells. Based on these findings, we performed subsequent experiments to determine whether oxaliplatin affected survival in response to gemcitabine and radiation.
Figure 1.
Combination indices of gemcitabine and oxaliplatin. Panc-1 cells were treated with various concentrations of gemcitabine or oxaliplatin at a fixed ratio of 1 gemcitabine to 10 oxaliplatin according to schedule 1 or 2. The combination indices were then calculated as described in the Materials and Methods section at surviving fractions between 0.5 and 0.01. Data are the mean of n = 3 to 5 independent experiments.
Table 1.
Combination Indices of Gemcitabine and Oxaliplatin at Surviving Fractions of 0.1 and 0.03.
| Fraction Unaffected | 0.1 | 0.03 |
| BxPC-3 | ||
| Schedule 1 | 1.22 ± 0.22 | 0.76 ± 0.05* |
| Schedule 2 | 1.12 ± 0.44 | 1.64 ± 0.49 |
| Panc-1 | ||
| Schedule 1 | 0.91 ± 0.10 | 0.83 ± 0.09 |
| Schedule 2 | 0.65 ± 0.15 | 0.61 ± 0.11* |
Data are the mean of n = 3 to 5 independent experiments ± standard error.
P < .05, statistically significant difference from 1.
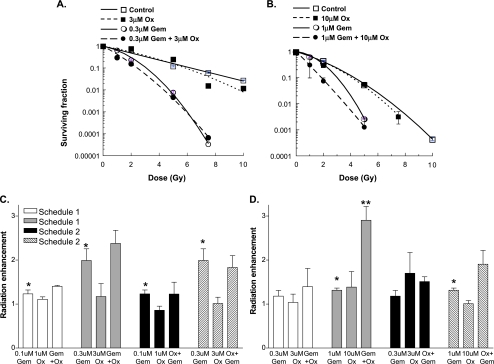
It was important to determine whether the combination of oxaliplatin with gemcitabine produced radiosensitization at least equal to that of gemcitabine alone. Because radiosensitization by gemcitabine occurs over a range of concentrations, we examined two different levels of cytotoxicity. In BxPC-3 cells treated under schedule 1 and low drug doses (0.1 µM Gem, 1 µM Ox), gemcitabine alone or in combination with oxaliplatin produced modest radiosensitization (enhancement ratios 1.2 ± 0.09 and 1.4 ± 0.02, respectively; Figure 2C and Table 2). Radiosensitization was dose-dependent as a higher gemcitabine concentration (0.3 µM) either alone or in combination with oxaliplatin (3 µM) produced greater radiosensitization under both drug treatment schedules 1 and 2 (Figure 2, A and C). It is important to note that oxaliplatin did not decrease gemcitabine-radiosensitization, regardless of exposure condition. In the Panc-1 cell line, gemcitabine alone produced only modest radiosensitization (Figure 2, B and D). However, the addition of oxaliplatin (10 µM) to gemcitabine under schedules 1 and 2 produced a marked increase in radiosensitization. Cytotoxicity did not appear to contribute to radiosensitization, as oxaliplatin alone produced cytotoxicity without radiosensitization. Taken together, these results show that oxaliplatin does not diminish gemcitabine-radiosensitization and that oxaliplatin can enhance gemcitabineradiosensitization under some conditions.
Figure 2.
The effects of gemcitabine and oxaliplatin on radiosensitization. (A and C) BxPC-3 or (B and D) Panc-1 cells were treated with the indicated concentrations of gemcitabine (Gem) or oxaliplatin (Ox) according to (A–D) schedule 1 or (C and D) schedule 2. Data are from a single representative experiment for each cell type with each condition performed in triplicate (A and B). Alternatively, the mean radiation enhancement ± standard error for three to six independent experiments is shown (C and D). Statistically significant differences from radiation alone (equal to 1) (*) or gemcitabine plus radiation (**) are indicated, where P < .05.
Table 2.
Radiosensitization and Cytotoxicity in Response to Gemcitabine and Oxaliplatin.
| Condition | Radiation Enhancement | Surviving Fraction |
| BxPC-3 | ||
| 0.1 µM Gem, schedule 1 | 1.23 ± 0.09 | 0.62 ± 0.13 |
| 1 µM Ox, schedule 1 | 1.11 ± 0.06 | 0.91 ± 0.01 |
| Gem + Ox, schedule 1 | 1.40 ± 0.02 | 0.81 ± 0.28 |
| 0.3 µM Gem, schedule 1 | 1.99 ± 0.27 | 0.39 ± 0.08 |
| 3 µM Ox, schedule 1 | 1.17 ± 0.29 | 0.59 ± 0.06 |
| Gem + Ox, schedule 1 | 2.38 ± 0.30 | 0.14 ± 0.02 |
| 0.1 µM Gem, schedule 2 | 1.23 ± 0.09 | 0.62 ± 0.13 |
| 1 µM Ox, schedule 2 | 0.86 ± 0.09 | 0.80 ± 0.23 |
| Gem + Ox, schedule 2 | 1.23 ± 0.27 | 0.30 ± 0.12 |
| 0.3 µM Gem, schedule 2 | 1.99 ± 0.27 | 0.39 ± 0.08 |
| 3 µM Ox, schedule 2 | 1.01 ± 0.14 | 0.65 ± 0.24 |
| Gem + Ox, schedule 2 | 1.83 ± 0.27 | 0.45 ± 0.02 |
| Panc-1 | ||
| 0.3 µM Gem, schedule 1 | 1.18 ± 0.13 | 1.07 ± 0.23 |
| 3 µM Ox, schedule 1 | 1.04 ± 0.19 | 0.76 ± 0.28 |
| Gem + Ox, schedule 1 | 1.39 ± 0.42 | 0.57 ± 0.14 |
| 1 µM Gem, schedule 1 | 1.31 ± 0.05 | 0.71 ± 0.07 |
| 10 µM Ox, schedule 1 | 1.38 ± 0.35 | 0.08 ± 0.05 |
| Gem + Ox, schedule 1 | 2.90 ± 0.31 | 0.04 ± 0.02 |
| 0.3 µM Gem, schedule 2 | 1.18 ± 0.13 | 1.07 ± 0.23 |
| 3 µM Ox, schedule 2 | 1.70 ± 0.47 | 0.77 ± 0.16 |
| Gem + Ox, schedule 2 | 1.51 ± 0.11 | 0.80 ± 0.08 |
| 1 µM Gem, schedule 2 | 1.31 ± 0.05 | 0.71 ± 0.07 |
| 10 µM Ox, schedule 2 | 1.01 ± 0.07 | 0.18 ± 0.00 |
| Gem + Ox, schedule 2 | 1.91 ± 0.32 | 0.21 ± 0.03 |
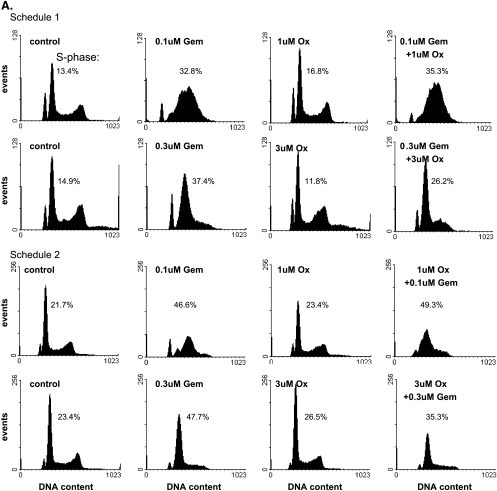
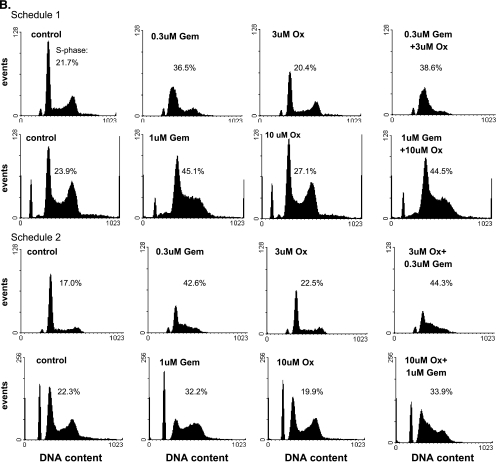
Given the importance of early S-phase cell cycle arrest in gemcitabine- mediated radiosensitization [16], we next analyzed the effects of gemcitabine and oxaliplatin on cell cycle distribution. In BxPC-3 cells, early S-phase accumulation was observed 24 hours after gemcitabine exposure, with higher concentrations of gemcitabine (0.3 µM) producing a sharper early S-phase arrest (Figure 3A). Oxaliplatin alone under either treatment schedule did not produce any changes in cell cycle distribution. In addition, the combination of oxaliplatin with gemcitabine in either sequence (schedule 1 or 2) produced cell cycle effects similar to gemcitabine alone. These observations in the BxPC-3 cells were further supported by similar results in the Panc-1 cells where gemcitabine produced early S-phase arrest that was not affected by oxaliplatin (Figure 3B). These data demonstrate that oxaliplatin treatment does not abrogate the early S-phase arrest in response to gemcitabine. Furthermore, these findings suggest that whereas the early S-phase arrest produced by gemcitabine is related to radiosensitization, cell cycle alterations do not account for the increased radiosensitization observed in response to gemcitabine and oxaliplatin.
Figure 3.
The effects of gemcitabine and oxaliplatin on cell cycle distribution. (A) BxPC-3 or (B) Panc-1 cells were treated with the indicated concentrations of gemcitabine (Gem) or oxaliplatin (Ox) according to schedule 1 or 2, as indicated. At the end of the drug treatment schedules cells were analyzed for DNA content. The percentage of cells in S-phase is indicated. Human lymphocytes were included as an internal control. Data shown are from a single experiment representative of at least three independent experiments.
To explore the mechanism underlying radiosensitization in response to gemcitabine and oxaliplatin, we assessed the effects on radiationinduced DNA damage response. The levels of γ-H2AX were used as a surrogate for unrepaired DNA damage. In these experiments, BxPC-3 or Panc-1 cells treated with gemcitabine and/or oxaliplatin (schedule 1) were irradiated and the γ-H2AX remaining 24 hours after radiation was determined. We found in BxPC-3 cells that γ-H2AX was elevated in response to each of the drugs alone as well as radiation alone (Figure 4A). The combination of gemcitabine and/or oxaliplatin with radiation did not cause persistence of γ-H2AX. In contrast, in the Panc-1 cells, we only observed elevation of residual γ-H2AX in response to gemcitabine (alone or in combination with oxaliplatin or radiation) (Figure 4B). These results demonstrate that a 2-hour exposure to gemcitabine produces a prolonged DNA damage response [17]. However, it did not appear that gemcitabine or oxaliplatin affected the γ-H2AX in response to radiation suggesting that radiosensitization by gemcitabine/oxaliplatin is mediated by mechanisms other than inhibition of DNA repair.
Figure 4.
The effects of gemcitabine and oxaliplatin on γ-H2AX in response to radiation. (A) BxPC-3 or (B) Panc-1 cells were treated with gemcitabine (0.3 or 1 µ M, respectively) or oxaliplatin (3 or 10 µM, respectively) according to treatment schedule 1. At the end of the treatment schedule, cells were treated with 8-Gy radiation. Twenty-four hours postirradiation, cells were analyzed for γ-H2AX by immunoblot analysis. Data are shown are from a single experiment representative of three independent experiments.
Discussion
In this study, we have found that the addition of oxaliplatin to gemcitabine-based chemoradiation produces radiosensitization equal to or greater than gemcitabine alone in a pancreatic cancer model. Because the majority of pancreatic cancer treatment failures after gemcitabine- radiotherapy are due to systemic disease progression and gemcitabine-oxaliplatin chemotherapy has already been shown superior to gemcitabine alone for metastatic disease [9], we designed a preclinical study within the context of our clinical goal of improving systemic disease control while maintaining local control (radiosensitization). We found that gemcitabine and oxaliplatin could produce synergistic cytotoxicity in pancreatic cancer cells. The addition of oxaliplatin to gemcitabine- radiation did not compromise radiosensitization by gemcitabine and, in some conditions, enhanced it. These findings support the clinical investigation of oxaliplatin in combination with gemcitabine-radiotherapy for locally advanced pancreatic cancer.
A number of efforts have been made to improve gemcitabine-based chemoradiotherapy for pancreatic cancer by adding other chemotherapeutic agents. In our previous work, we conducted preclinical and clinical studies combining cisplatin with gemcitabine-radiation. This Phase I trial suggested that the addition of cisplatin to gemcitabine-radiation therapy might improve overall survival (13 months) however patients experienced increased toxicity [8]. Some trials have combined 5-FU with gemcitabine-radiotherapy producing unacceptable toxicity [18] but in others using a lower dose of radiation (45–50 Gy) and smaller fields producing promising results with median survivals of 14 months [19]. The combination of paclitaxel with gemcitabine-radiotherapy has also been tested in Phase I clinical trials [20] and Phase II trials are underway. Irinotecan has been used in an effort to improve on gemcitabine-radiotherapy. The addition of induction irinotecan to gemcitabine-radiotherapy produced only a slight improvement in overall survival [21]. Together, these studies indicate that combining additional chemotherapeutic agents with gemcitabine-radiation can increase the therapeutic efficacy if additional toxicity can be avoided.
Molecularly targeted agents are attractive candidates for combining with gemcitabine-radiation because they typically do not possess the cytotoxicity associated with standard chemotherapeutic agents. Recently, a randomized Phase III clinical trial in pancreatic cancer demonstrated a modest but statistically significant improvement in overall survival of 6.2 vs 5.9 months in patients treated with gemcitabine plus erlotinib versus gemcitabine alone [22]. The results of this trial support the clinical investigation of erlotinib with gemcitabine- radiation. A Phase I study of gemcitabine- and paclitaxel-based chemoradiation with erlotinib has recently been completed and demonstrated promising activity with a median survival of 14 months [23].
Whereas the majority of gemcitabine-radiation treatment failures are due to systemic disease progression, local tumor control is still critical in the management of pancreatic cancer both in terms of palliative care as well as survival [3]. Although within the context of the present study we have focused on systemic disease control, local tumor control should not be overlooked. As new treatment strategies increase the efficacy of systemic disease control, local tumor control will become a greater issue. Given the severe toxicity of full-dose chemotherapy with large field radiation [24–26], it seems reasonable to investigate the use of IMRT with smaller radiation fields to optimize local tumor control when combining new agents with gemcitabine-radiation.
Based on the present study and others [9], we have initiated a clinical trial combining oxaliplatin with gemcitabine-radiotherapy for patients with pancreatic cancer. This trial is designed to determine the dose of oxaliplatin that can be added to full-dose gemcitabine and radiation therapy. The initial results of this study indicate that oxaliplatin can be safely combined with gemcitabine-radiation with promising efficacy, but the determination of the efficacy of this approach will require further study.
Footnotes
This work was supported by grant no. CA78554 and by the University of Michigan Cancer Center support grant no. 5 P30 CA46592.
References
- 1.McGinn CJ, Zalupski MM, Shureiqi I, Robertson JM, Eckhauser FE, Smith DC, Brown D, Hejna G, Strawderman M, Normolle D, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 2.Storniolo AM, Enas NH, Brown CA, Voi M, Rothenberg ML, Schilsky R. An investigational new drug treatment program for patients with gemcitabine: results for over 3000 patients with pancreatic carcinoma. Cancer. 1999;85:1261–1268. [PubMed] [Google Scholar]
- 3.Murphy JD, Adusumilli S, Griffith KA, Ray ME, Zalupski MM, Lawrence TS, Ben-Josef E. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2007;68:801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34:867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Frytak S, Hahn RG, O'Connell MJ, Reitemeier RJ, Rubin J, Schutt AJ, Weiland LH, Childs DS, Holbrook MA, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Philip PA, Zalupski MM, Vaitkevicius VK, Arlauskas P, Chaplen R, Heilbrun LK, Adsay V, Weaver D, Shields AF. Phase II study of gemcitabine and cisplatin in the treatment of patients with advanced pancreatic carcinoma. Cancer. 2001;92:569–577. doi: 10.1002/1097-0142(20010801)92:3<569::aid-cncr1356>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Symon Z, Davis M, McGinn CJ, Zalupski MM, Lawrence TS. Concurrent chemoradiotherapy with gemcitabine and cisplatin for pancreatic cancer: from the laboratory to the clinic. Int J Radiat Oncol Biol Phys. 2002;53:140–145. doi: 10.1016/s0360-3016(01)02790-0. [DOI] [PubMed] [Google Scholar]
- 8.Muler JH, McGinn CJ, Normolle D, Lawrence T, Brown D, Hejna G, Zalupski MM. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22:238–243. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 9.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, Zaniboni A, Ducreux M, Aitini E, Taieb J, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence TS. Ouabain sensitizes tumor cells but not normal cells to radiation. Int J Radiat Oncol Biol Phys. 1988;15:953–958. doi: 10.1016/0360-3016(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 11.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 12.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Heinemann V, Hertel LW, Grindey GB, Plunkett W. Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluorodeoxycytidine and 1-beta-d-arabinofuranosylcytosine. Cancer Res. 1988;48:4024–4031. [PubMed] [Google Scholar]
- 14.Woynarowski JM, Faivre S, Herzig MC, Arnett B, Chapman WG, Trevino AV, Raymond E, Chaney SG, Vaisman A, Varchenko M, et al. Oxaliplatin-induced damage of cellular DNA. Mol Pharmaco. 2000;l58:920–927. doi: 10.1124/mol.58.5.920. [DOI] [PubMed] [Google Scholar]
- 15.Pappas P, Mavroudis D, Nikolaidou M, Georgoulias V, Marselos M. Coadministration of oxaliplatin does not influence the pharmacokinetics of gemcitabine. Anticancer Drugs. 2006;17:1185–1191. doi: 10.1097/01.cad.0000236303.97467.49. [DOI] [PubMed] [Google Scholar]
- 16.Morgan MA, Parsels LA, Parsels JD, Mesiwala AK, Maybaum J, Lawrence TS. Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res. 2005;65:6835–6842. doi: 10.1158/0008-5472.CAN-04-2246. [DOI] [PubMed] [Google Scholar]
- 17.Ewald B, Sampath D, Plunkett W. H2AX phosphorylation marks gemcitabine-induced stalled replication forks and their collapse upon S-phase checkpoint abrogation. Mol Cancer Ther. 2007;6:1239–1248. doi: 10.1158/1535-7163.MCT-06-0633. [DOI] [PubMed] [Google Scholar]
- 18.Talamonti MS, Catalano PJ, Vaughn DJ, Whittington R, Beauchamp RD, Berlin J, Benson AB., III Eastern Cooperative Oncology Group phase I trial of protracted venous infusion fluorouracil plus weekly gemcitabine with concurrent radiation therapy in patients with locally advanced pancreas cancer: a regimen with unexpected early toxicity. J Clin Oncol. 2000;18:3384–3389. doi: 10.1200/JCO.2000.18.19.3384. [DOI] [PubMed] [Google Scholar]
- 19.Wilkowski R, Thoma M, Bruns C, Wagner A, Heinemann V. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP. 2006;7:349–360. [PubMed] [Google Scholar]
- 20.Safran H, Dipetrillo T, Iannitti D, Quirk D, Akerman P, Cruff D, Cioffi W, Shah S, Ramdin N, Rich T. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a phase I trial. Int J Radiat Oncol Biol Phys. 2002;54:137–141. doi: 10.1016/s0360-3016(02)02902-4. [DOI] [PubMed] [Google Scholar]
- 21.Mishra G, Butler J, Ho C, Melin S, Case LD, Ennever PR, Magrinat GC, Bearden JD, Minotto DC, Howerton R, et al. Phase II trial of inductio gemcitabine/CPT-11 followed by a twice-weekly infusion of gemcitabine and concurrent external beam radiation for the treatment of locally advanced pancreatic cancer. Am J Clin Oncol. 2005;28:345–350. doi: 10.1097/01.coc.0000159559.42311.c5. [DOI] [PubMed] [Google Scholar]
- 22.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 23.Iannitti D, Dipetrillo T, Akerman P, Barnett JM, Maia-Acuna C, Cruff D, Miner T, Martel D, Cioffi W, Remis M, et al. Erlotinib and chemoradiation followed by maintenance erlotinib for locally advanced pancreatic cancer: a phase I study. Am J Clin Oncol. 1999;28:570–575. doi: 10.1097/01.coc.0000184682.51193.00. [DOI] [PubMed] [Google Scholar]
- 24.Crane CH, Janjan NA, Evans DB, Wolff RA, Ballo MT, Milas L, Mason K, Charnsangavej C, Pisters PW, Lee JE, et al. Toxicity and efficacy of concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Int J Pancreatol. 2001;29:9–18. doi: 10.1385/IJGC:29:1:09. [DOI] [PubMed] [Google Scholar]
- 25.Crane CH, Antolak JA, Rosen II, Forster KM, Evans DB, Janjan NA, Charnsangavej C, Pisters PW, Lenzi R, Papagikos MA, et al. Phase I study of concomitant gemcitabine and IMRT for patients with unresectable adenocarcinoma of the pancreatic head. Int J Gastrointest Cancer. 2001;30:123–132. doi: 10.1385/IJGC:30:3:123. [DOI] [PubMed] [Google Scholar]
- 26.Scalliet P, Goor C, Galdermans D, Meerbeek JV, Groen HJ, Leest AHDVd, Westerink H, Jungnelius U, Turrisi L. Gemcitabine with thoracic radiotherapy—a phase II pilot study in chemonaive patients with advanced non-small cell lung cancer. Proc Am Soc Clin Oncol. 1998;17:499a. Abstract # 1923. [Google Scholar]