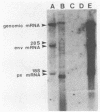
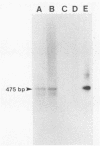
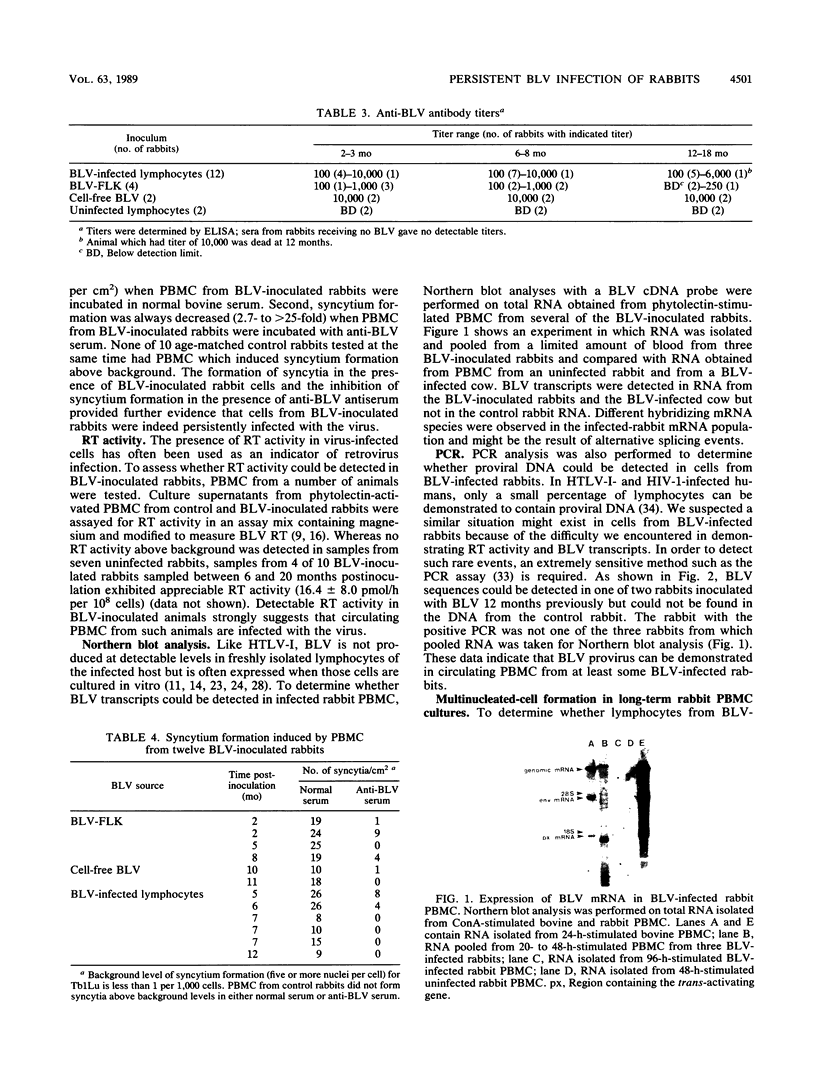
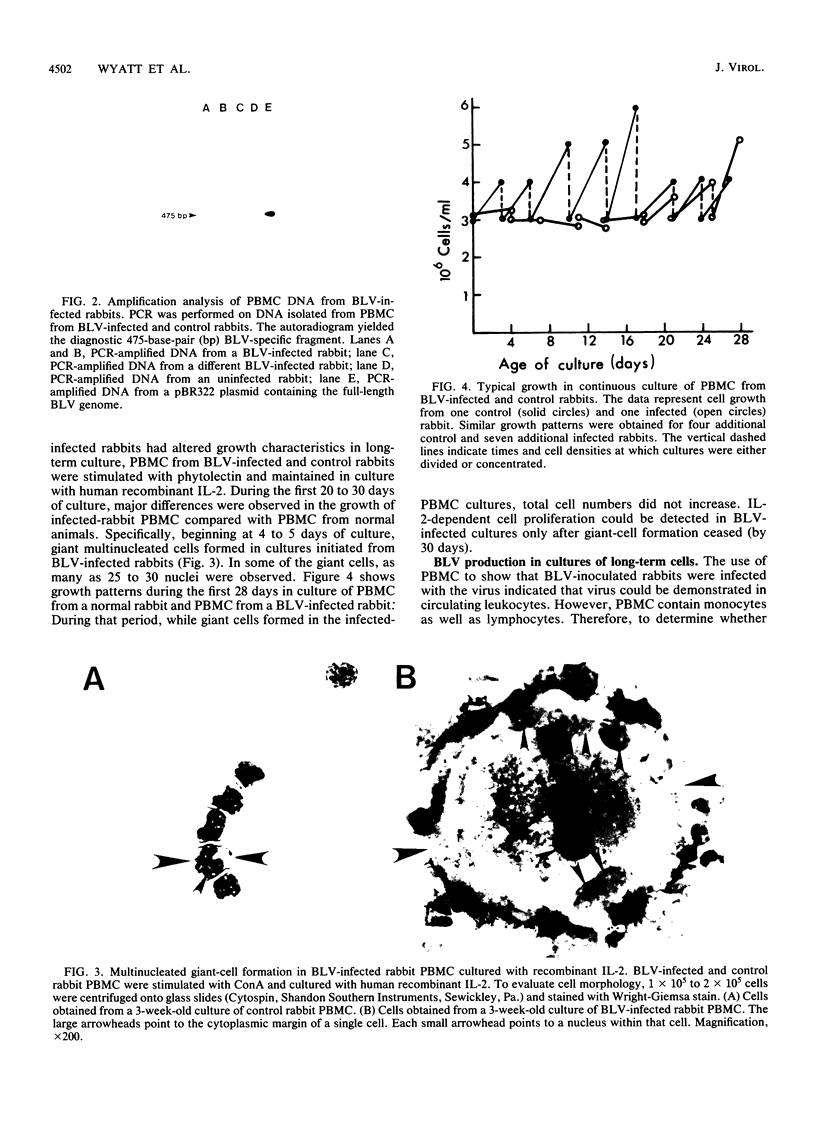
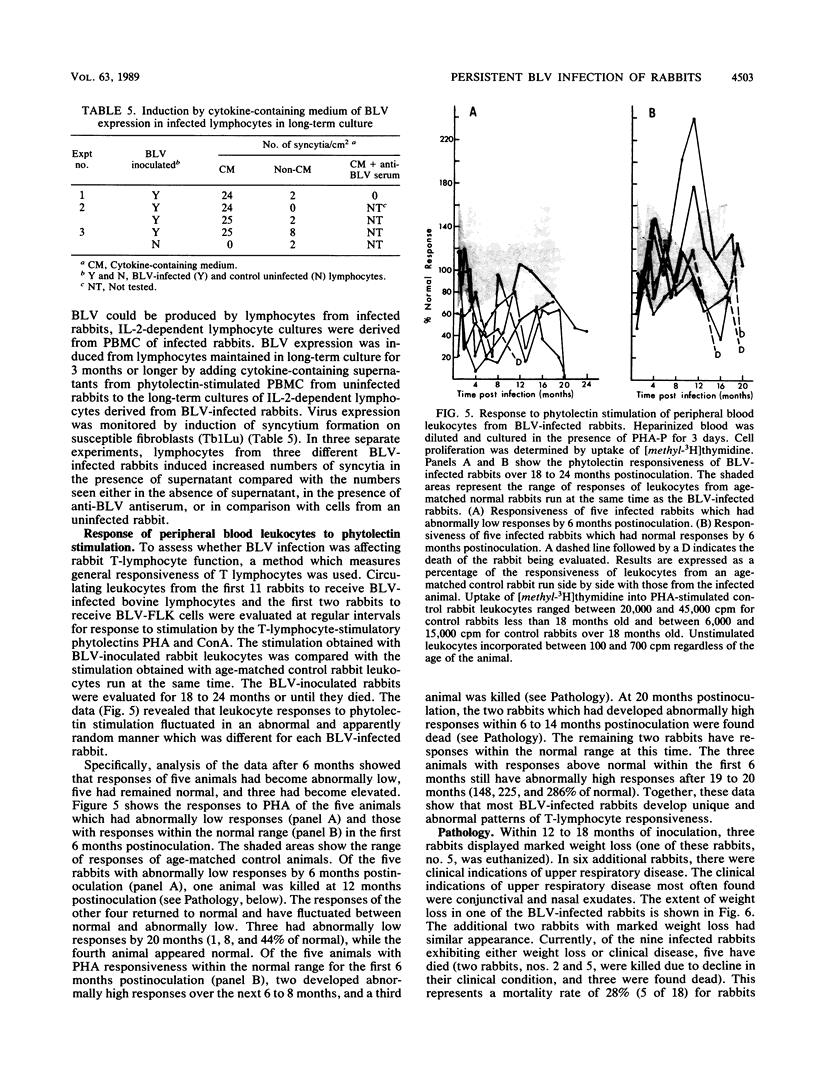
Abstract
Bovine leukemia virus (BLV) infection of rabbits provides a safe and relatively inexpensive in vivo mammalian system for the study of the mechanisms controlling expression of a unique group of lymphotropic retroviruses. This group of viruses, which includes C-type human T-lymphotropic virus types I and II and lentiviruslike human immunodeficiency virus type 1, possesses genes coding for "trans-activating" products. Rabbits experimentally inoculated with BLV became persistently infected, as demonstrated by a number of tests. All BLV-inoculated rabbits developed persistent serum antibody to BLV. Furthermore, all BLV-inoculated rabbits had peripheral blood mononuclear cells which, when stimulated, expressed the virus, as demonstrated by viral induction of syncytium formation in a BLV-susceptible fibroblast line. The presence of BLV in circulating cells was confirmed by using peripheral blood mononuclear cells from randomly selected BLV-inoculated rabbits, which showed the presence of viral reverse transcriptase activity, BLV transcriptional activity, or BLV proviral DNA. Additional tests showed that infected lymphocytes maintained in culture with recombinant human interleukin-2 formed multinucleated giant cells and produced virus when incubated in cytokine-containing medium. BLV-infected rabbits also showed alterations in several parameters associated with immunity, beginning 6 months after inoculation. Thirty-eight percent of infected rabbits developed abnormally low T-cell responses, as measured by phytolectin stimulation, and T-cell responses cycled between normal and abnormally low over a period of 20 to 24 months. Forty-four percent of rabbits infected for longer than 12 months suffered from recurrent conjunctivitis and rhinitis. By 24 months postinoculation, 28% of infected rabbits were dead or were killed because of poor clinical condition.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baliga V., Ferrer J. F. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc Soc Exp Biol Med. 1977 Nov;156(2):388–391. doi: 10.3181/00379727-156-39942. [DOI] [PubMed] [Google Scholar]
- Bouillant A. M., Becker S. A. Ultrastructural comparison of Oncovirinae (type C), Spumavirinae, and Lentivirinae: three subfamilies of Retroviridae found in farm animals. J Natl Cancer Inst. 1984 May;72(5):1075–1084. [PubMed] [Google Scholar]
- Buck C., McKeirnan A., Evermann J., Magnuson N. S., Reeves R. A rapid method for the large scale preparation of bovine leukemia virus antigen. Vet Microbiol. 1988 Jun;17(2):107–116. doi: 10.1016/0378-1135(88)90002-8. [DOI] [PubMed] [Google Scholar]
- Derse D. trans-acting regulation of bovine leukemia virus mRNA processing. J Virol. 1988 Apr;62(4):1115–1119. doi: 10.1128/jvi.62.4.1115-1119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diglio C. A., Ferrer J. F. Induction of syncytia by the bovine C-type leukemia virus. Cancer Res. 1976 Mar;36(3):1056–1067. [PubMed] [Google Scholar]
- Epstein J. S., Frederick W. R., Rook A. H., Jackson L., Manischewitz J. F., Mayner R. E., Masur H., Enterline J. C., Djeu J. Y., Quinnan G. V., Jr Selective defects in cytomegalovirus- and mitogen-induced lymphocyte proliferation and interferon release in patients with acquired immunodeficiency syndrome. J Infect Dis. 1985 Oct;152(4):727–733. doi: 10.1093/infdis/152.4.727. [DOI] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Diglio C. A. Development of an in vitro infectivity assay for the C-type bovine leukemia virus. Cancer Res. 1976 Mar;36(3):1068–1073. [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysdael J., Bruck C., Kettmann R., Burny A. Bovine leukemia virus. Curr Top Microbiol Immunol. 1984;112:1–19. doi: 10.1007/978-3-642-69677-0_1. [DOI] [PubMed] [Google Scholar]
- Gilden R. V., Long C. W., Hanson M., Toni R., Charman H. P., Oroszlan S., Miller J. M., Van der Maaten M. J. Characteristics of the major internal protein and RNA-dependent DNA polymerase of bovine leukaemia virus. J Gen Virol. 1975 Dec;29(3):305–314. doi: 10.1099/0022-1317-29-3-305. [DOI] [PubMed] [Google Scholar]
- Gluckman J. C., Klatzmann D., Montagnier L. Lymphadenopathy-associated-virus infection and acquired immunodeficiency syndrome. Annu Rev Immunol. 1986;4:97–117. doi: 10.1146/annurev.iy.04.040186.000525. [DOI] [PubMed] [Google Scholar]
- Gonda M. A., Braun M. J., Carter S. G., Kost T. A., Bess J. W., Jr, Arthur L. O., Van der Maaten M. J. Characterization and molecular cloning of a bovine lentivirus related to human immunodeficiency virus. 1987 Nov 26-Dec 2Nature. 330(6146):388–391. doi: 10.1038/330388a0. [DOI] [PubMed] [Google Scholar]
- Graves D. C., Diglio C. A., Ferrer J. F. A reverse transcriptase assay for detection of the bovine leukemia virus. Am J Vet Res. 1977 Nov;38(11):1739–1744. [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Sagata N., Ikawa Y. The bovine leukemia virus X region encodes a trans-activator of its long terminal repeat. Jpn J Cancer Res. 1987 Feb;78(2):93–98. [PubMed] [Google Scholar]
- Kenyon S. J., Ferrer J. F., McFeely R. A., Graves D. C. Induction of lymphosarcoma in sheep by bovine leukemia virus. J Natl Cancer Inst. 1981 Nov;67(5):1157–1163. [PubMed] [Google Scholar]
- Kettmann R., Deschamps J., Cleuter Y., Couez D., Burny A., Marbaix G. Leukemogenesis by bovine leukemia virus: proviral DNA integration and lack of RNA expression of viral long terminal repeat and 3' proximate cellular sequences. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2465–2469. doi: 10.1073/pnas.79.8.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettmann R., Marbaix G., Cleuter Y., Portetelle D., Mammerickx M., Burny A. Genomic integration of bovine leukemia provirus and lack of viral RNA expression in the target cells of cattle with different responses to BLV infection. Leuk Res. 1980;4(6):509–519. doi: 10.1016/0145-2126(80)90062-4. [DOI] [PubMed] [Google Scholar]
- Lagarias D. M., Radke K. Transcriptional activation of bovine leukemia virus in blood cells from experimentally infected, asymptomatic sheep with latent infections. J Virol. 1989 May;63(5):2099–2107. doi: 10.1128/jvi.63.5.2099-2107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt L. J., Reyes G. R., Moonka D. K., Bensch K., Miller R. A., Engleman E. G. Human T cell leukemia virus-I-associated T-suppressor cell inhibition of erythropoiesis in a patient with pure red cell aplasia and chronic T gamma-lymphoproliferative disease. J Clin Invest. 1988 Feb;81(2):538–548. doi: 10.1172/JCI113352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Montefiori D. C., Mitchell W. M. Persistent coinfection of T lymphocytes with HTLV-II and HIV and the role of syncytium formation in HIV-induced cytopathic effect. Virology. 1987 Oct;160(2):372–378. doi: 10.1016/0042-6822(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Olson C., Kettmann R., Burny A., Kaja R. Goat lymphosarcoma from bovine leukemia virus. J Natl Cancer Inst. 1981 Sep;67(3):671–675. [PubMed] [Google Scholar]
- Oste C. Polymerase chain reaction. Biotechniques. 1988 Feb;6(2):162–167. [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Paul P. S., Pomeroy K. A., Castro A. E., Johnson D. W., Muscoplat C. C., Sorensen D. K. Detection of bovine leukemia virus in B-lymphocytes by the syncytia induction assay. J Natl Cancer Inst. 1977 Oct;59(4):1269–1272. doi: 10.1093/jnci/59.4.1269. [DOI] [PubMed] [Google Scholar]
- Sentsui H., Kono Y., Itohara S., Ishino S. Experimental infection of bovine leukemia virus in small laboratory animals. Nihon Juigaku Zasshi. 1988 Dec;50(6):1245–1251. doi: 10.1292/jvms1939.50.1245. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Stock N. D., Ferrer J. F. Replicating C-type virus in phytohemagglutinin-treated buffy-coat cultures of bovine origin. J Natl Cancer Inst. 1972 Apr;48(4):985–996. [PubMed] [Google Scholar]
- Van der Maaten M. J., Boothe A. D., Seger C. L. Isolation of a virus from cattle with persistent lymphocytosis. J Natl Cancer Inst. 1972 Dec;49(6):1649–1657. doi: 10.1093/jnci/49.6.1649. [DOI] [PubMed] [Google Scholar]
- Walstra K., Gratwohl A., Riederer I., Speck B. B/T cell ratio of rabbit peripheral blood lymphocytes. Influence of separation technique on results. J Immunol Methods. 1985 May 10;79(1):143–147. doi: 10.1016/0022-1759(85)90400-4. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Yoffe B., Lewis D. E., Petrie B. L., Noonan C. A., Melnick J. L., Hollinger F. B. Fusion as a mediator of cytolysis in mixtures of uninfected CD4+ lymphocytes and cells infected by human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1429–1433. doi: 10.1073/pnas.84.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]