Abstract
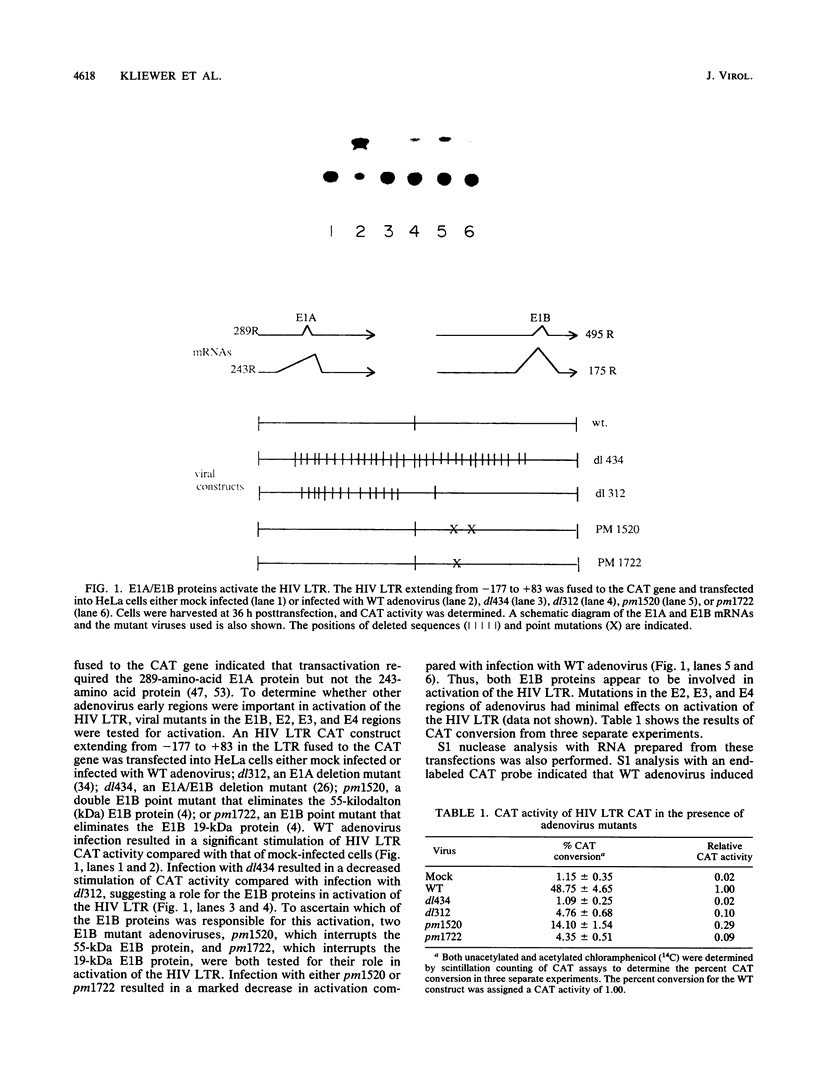
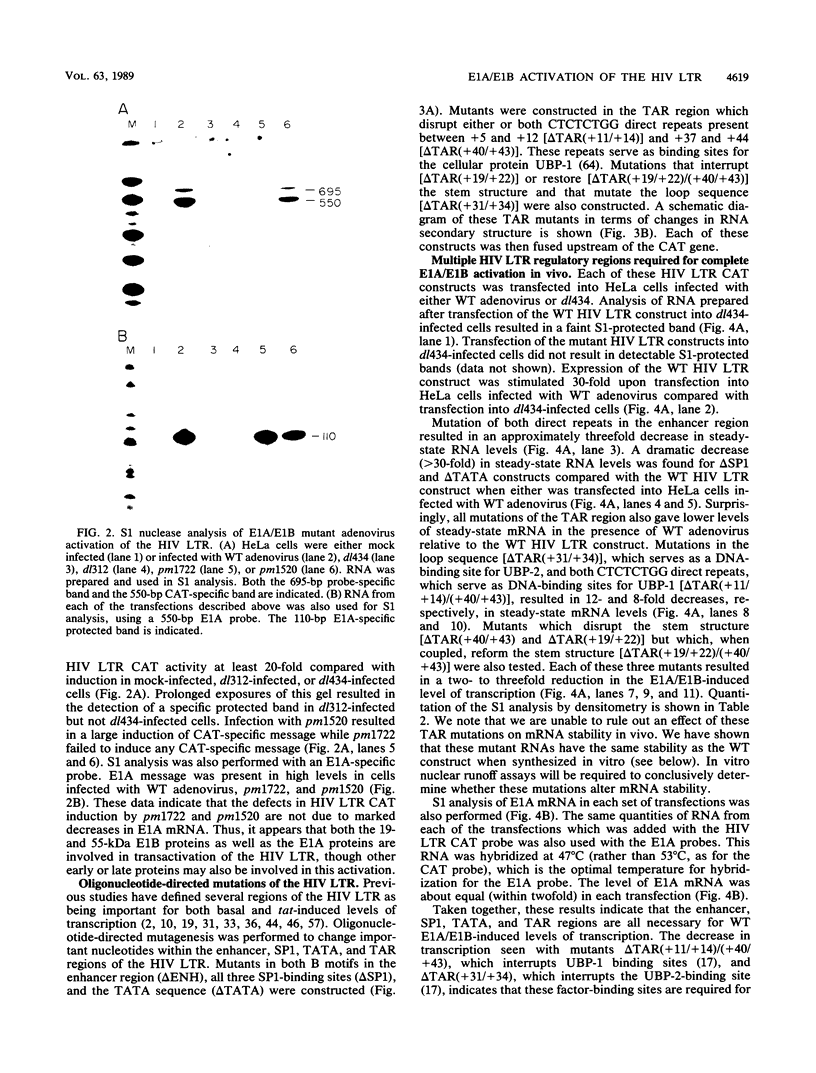
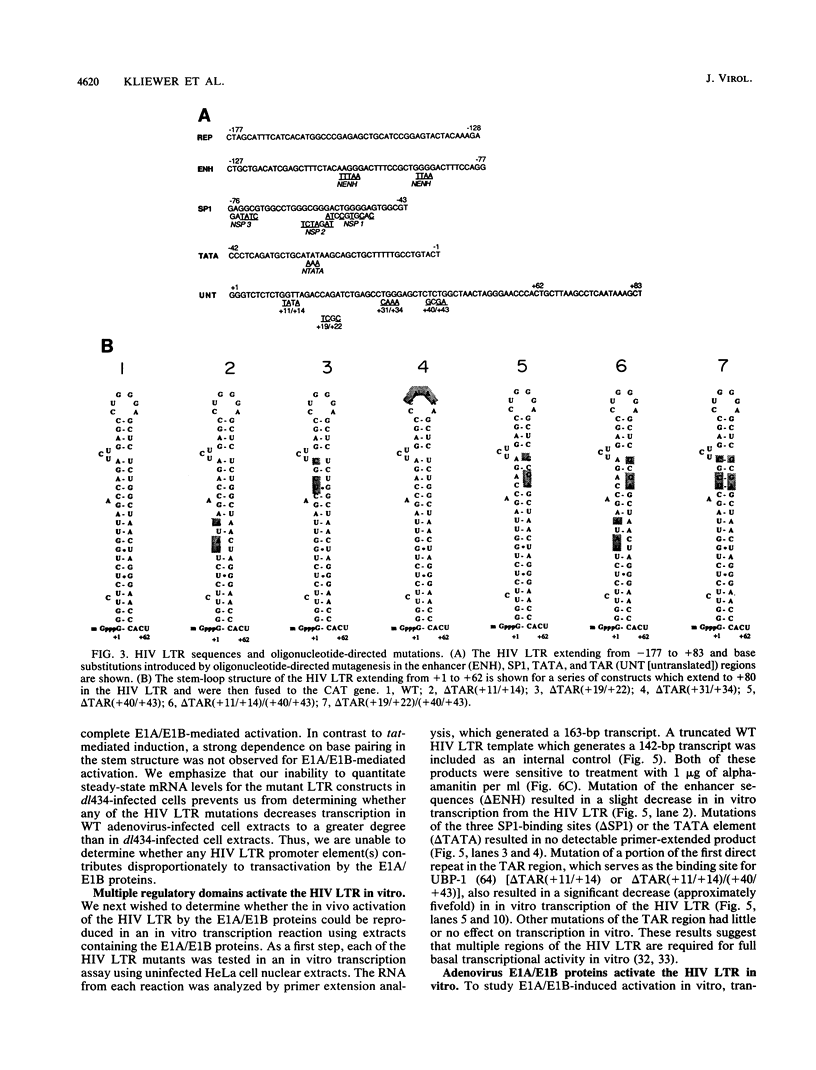
The human immunodeficiency virus (HIV) type 1 long terminal repeat (LTR) is the site of activation of the HIV tat protein. However, additional transactivators, such as the adenovirus E1A and herpesvirus ICPO proteins, have also been shown to be capable of activating the HIV LTR. Analysis of adenovirus mutants indicated that complete transactivation of the HIV LTR was dependent on both the E1A and E1B proteins. To determine which regions of the HIV LTR were important for complete E1A/E1B activation, a variety of oligonucleotide-directed mutations in HIV transcriptional regulatory domains were assayed both in vivo and in vitro. S1 nuclease analysis of RNA prepared after transfection of these HIV constructs into HeLa cells infected with wild-type adenovirus indicated that the enhancer, SP1, TATA, and a portion of the transactivation-responsive element were each required for complete E1A/E1B-mediated activation of the HIV LTR. These same promoter elements were required for both basal and E1A/E1B-induced levels of transcription in in vitro transcription reactions performed with cellular extracts prepared from cells infected with dl434, an E1A/E1B deletion mutant, or wild-type adenovirus. No mutations were found that reduced only E1A/E1B-induced expression without proportionally reducing basal levels of transcription, suggesting that E1A/E1B-mediated induction of the HIV LTR requires multiple promoter elements which are also required for basal transcriptional levels. Unlike activation by the tat protein, there was not a rigid dependence on maintenance of the transactivation-responsive stem base pairing for E1A/E1B-mediated activation either in vivo or in vitro, indicating that activation occurs by a mechanism distinct from that of tat induction.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N., Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988 Sep 16;241(4872):1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Barker D. D., Berk A. J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987 Jan;156(1):107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Culp J. S., Webster L. C., Friedman D. J., Smith C. L., Huang W. J., Wu F. Y., Rosenberg M., Ricciardi R. P. The 289-amino acid E1A protein of adenovirus binds zinc in a region that is important for trans-activation. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6450–6454. doi: 10.1073/pnas.85.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter H., Chiu R., Imagawa M., Karin M., Jones K. A. In vitro activation of the HIV-1 enhancer in extracts from cells treated with a phorbol ester tumor promoter. EMBO J. 1987 Dec 20;6(13):4067–4071. doi: 10.1002/j.1460-2075.1987.tb02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M. B., Jarrett R. F., Aldovini A., Gallo R. C., Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986 Sep 12;46(6):807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- Feng S., Holland E. C. HIV-1 tat trans-activation requires the loop sequence within tar. Nature. 1988 Jul 14;334(6178):165–167. doi: 10.1038/334165a0. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Feinberg M. B., Josephs S. F., Harper M. E., Marselle L. M., Reyes G., Gonda M. A., Aldovini A., Debouk C., Gallo R. C. The trans-activator gene of HTLV-III is essential for virus replication. 1986 Mar 27-Apr 2Nature. 320(6060):367–371. doi: 10.1038/320367a0. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Bredt D. S., Pabo C. O. Tat protein from human immunodeficiency virus forms a metal-linked dimer. Science. 1988 Apr 1;240(4848):70–73. doi: 10.1126/science.2832944. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science. 1984 May 4;224(4648):500–503. doi: 10.1126/science.6200936. [DOI] [PubMed] [Google Scholar]
- Garcia J. A., Harrich D., Pearson L., Mitsuyasu R., Gaynor R. B. Functional domains required for tat-induced transcriptional activation of the HIV-1 long terminal repeat. EMBO J. 1988 Oct;7(10):3143–3147. doi: 10.1002/j.1460-2075.1988.tb03181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. A., Harrich D., Soultanakis E., Wu F., Mitsuyasu R., Gaynor R. B. Human immunodeficiency virus type 1 LTR TATA and TAR region sequences required for transcriptional regulation. EMBO J. 1989 Mar;8(3):765–778. doi: 10.1002/j.1460-2075.1989.tb03437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. A., Wu F. K., Mitsuyasu R., Gaynor R. B. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 1987 Dec 1;6(12):3761–3770. doi: 10.1002/j.1460-2075.1987.tb02711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J., Wu F., Gaynor R. Upstream regulatory regions required to stabilize binding to the TATA sequence in an adenovirus early promoter. Nucleic Acids Res. 1987 Oct 26;15(20):8367–8385. doi: 10.1093/nar/15.20.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Hillman D., Berk A. J. Adenovirus early region 1A protein activates transcription of a nonviral gene introduced into mammalian cells by infection or transfection. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1193–1197. doi: 10.1073/pnas.81.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Tsukamoto A., Montell C., Berk A. J. Enhanced expression of adenovirus transforming proteins. J Virol. 1982 Oct;44(1):276–285. doi: 10.1128/jvi.44.1.276-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Phelps W., Feigenbaum L., Ostrove J. M., Adachi A., Howley P. M., Khoury G., Ginsberg H. S., Martin M. A. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. doi: 10.1073/pnas.83.24.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Klessig D. F. Expression of unselected adenovirus genes in human cells co-transformed with the HSV-1 tk gene and adenovirus 2 DNA. Cell. 1980 Sep;21(2):453–463. doi: 10.1016/0092-8674(80)90482-1. [DOI] [PubMed] [Google Scholar]
- Harrich D., Garcia J., Wu F., Mitsuyasu R., Gonazalez J., Gaynor R. Role of SP1-binding domains in in vivo transcriptional regulation of the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1989 Jun;63(6):2585–2591. doi: 10.1128/jvi.63.6.2585-2591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Cullen B. R. Mutational analysis of the trans-activation-responsive region of the human immunodeficiency virus type I long terminal repeat. J Virol. 1988 Mar;62(3):673–679. doi: 10.1128/jvi.62.3.673-679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Perkins A., Heimer E. P., Cullen B. R. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6364–6368. doi: 10.1073/pnas.84.18.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale M. J., Hart R. P., Nevins J. R. An enhancer-like element in the adenovirus E2 promoter contains sequences essential for uninduced and E1A-induced transcription. Proc Natl Acad Sci U S A. 1985 Jan;82(2):381–385. doi: 10.1073/pnas.82.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Smith D. H., Jakobovits E. B., Capon D. J. A discrete element 3' of human immunodeficiency virus 1 (HIV-1) and HIV-2 mRNA initiation sites mediates transcriptional activation by an HIV trans activator. Mol Cell Biol. 1988 Jun;8(6):2555–2561. doi: 10.1128/mcb.8.6.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Luciw P. A., Tjian R. Activation of the AIDS retrovirus promoter by the cellular transcription factor, Sp1. Science. 1986 May 9;232(4751):755–759. doi: 10.1126/science.3008338. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Luciw P. A., Duchange N. Structural arrangements of transcription control domains within the 5'-untranslated leader regions of the HIV-1 and HIV-2 promoters. Genes Dev. 1988 Sep;2(9):1101–1114. doi: 10.1101/gad.2.9.1101. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. Y., Calman A. F., Luciw P. A., Peterlin B. M. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature. 1987 Dec 3;330(6147):489–493. doi: 10.1038/330489a0. [DOI] [PubMed] [Google Scholar]
- Kaufman J. D., Valandra G., Roderiquez G., Bushar G., Giri C., Norcross M. A. Phorbol ester enhances human immunodeficiency virus-promoted gene expression and acts on a repeated 10-base-pair functional enhancer element. Mol Cell Biol. 1987 Oct;7(10):3759–3766. doi: 10.1128/mcb.7.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Scheidereit C., Roeder R. G. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-kappa B) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff T., Corden J., Elkaim R., Sassone-Corsi P. Transcriptional analysis of the adenovirus-5 EIII promoter: absence of sequence specificity for stimulation by EIa gene products. Nucleic Acids Res. 1985 Feb 25;13(4):1209–1221. doi: 10.1093/nar/13.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong K., Brunet L., Berk A. J. Factors responsible for the higher transcriptional activity of extracts of adenovirus-infected cells fractionate with the TATA box transcription factor. Mol Cell Biol. 1988 Apr;8(4):1765–1774. doi: 10.1128/mcb.8.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Cheng-Mayer C., Dina D., Luciw P. A. AIDS retrovirus (ARV-2) clone replicates in transfected human and animal fibroblasts. Science. 1986 May 23;232(4753):998–1001. doi: 10.1126/science.3010461. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hoffman A. D., Kramer S. M., Landis J. A., Shimabukuro J. M., Oshiro L. S. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984 Aug 24;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Hayward G. S., Pitha P. M. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7408–7412. doi: 10.1073/pnas.84.21.7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca J. D., Bednarik D. P., Raj N. B., Rosen C. A., Sodroski J. G., Haseltine W. A., Pitha P. M. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- Muesing M. A., Smith D. H., Capon D. J. Regulation of mRNA accumulation by a human immunodeficiency virus trans-activator protein. Cell. 1987 Feb 27;48(4):691–701. doi: 10.1016/0092-8674(87)90247-9. [DOI] [PubMed] [Google Scholar]
- Murthy S. C., Bhat G. P., Thimmappaya B. Adenovirus EIIA early promoter: transcriptional control elements and induction by the viral pre-early EIA gene, which appears to be sequence independent. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2230–2234. doi: 10.1073/pnas.82.8.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel G. J., Rice S. A., Knipe D. M., Baltimore D. Alternative mechanisms for activation of human immunodeficiency virus enhancer in T cells. Science. 1988 Mar 11;239(4845):1299–1302. doi: 10.1126/science.2830675. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Wong-Staal F. Demonstration of virus-specific transcriptional activator(s) in cells infected with HTLV-III by an in vitro cell-free system. Cell. 1986 Oct 10;47(1):29–35. doi: 10.1016/0092-8674(86)90363-6. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Leonard J., Weck K. E., Rabson A. B., Gendelman H. E. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3726–3732. doi: 10.1128/jvi.61.12.3726-3732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin N. T., Cohen E. A., Darveau A., Rosen C., Haseltine W., Sonenberg N. Mutational analysis of the 5' non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO J. 1988 Sep;7(9):2831–2837. doi: 10.1002/j.1460-2075.1988.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin B. M., Luciw P. A., Barr P. J., Walker M. D. Elevated levels of mRNA can account for the trans-activation of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9734–9738. doi: 10.1073/pnas.83.24.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Trans-activation of the human immunodeficiency virus long terminal repeat sequences, expressed in an adenovirus vector, by the adenovirus E1A 13S protein. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4200–4204. doi: 10.1073/pnas.85.12.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice A. P., Mathews M. B. Transcriptional but not translational regulation of HIV-1 by the tat gene product. Nature. 1988 Apr 7;332(6164):551–553. doi: 10.1038/332551a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Goh W. C., Dayton A. I., Lippke J., Haseltine W. A. Post-transcriptional regulation accounts for the trans-activation of the human T-lymphotropic virus type III. Nature. 1986 Feb 13;319(6054):555–559. doi: 10.1038/319555a0. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985 Jul;41(3):813–823. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Josephs S. F., Dukovich M., Peffer N., Wong-Staal F., Greene W. C. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Dayton A., Terwilliger E., Haseltine W. A second post-transcriptional trans-activator gene required for HTLV-III replication. Nature. 1986 May 22;321(6068):412–417. doi: 10.1038/321412a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- White E., Denton A., Stillman B. Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression. J Virol. 1988 Sep;62(9):3445–3454. doi: 10.1128/jvi.62.9.3445-3454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., McClanahan T. K., Morimoto R. I. E1a transactivation of the human HSP70 promoter is mediated through the basal transcriptional complex. Mol Cell Biol. 1989 Jun;9(6):2574–2587. doi: 10.1128/mcb.9.6.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. K., Garcia J. A., Harrich D., Gaynor R. B. Purification of the human immunodeficiency virus type 1 enhancer and TAR binding proteins EBP-1 and UBP-1. EMBO J. 1988 Jul;7(7):2117–2130. doi: 10.1002/j.1460-2075.1988.tb03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Garcia J., Mitsuyasu R., Gaynor R. Alterations in binding characteristics of the human immunodeficiency virus enhancer factor. J Virol. 1988 Jan;62(1):218–225. doi: 10.1128/jvi.62.1.218-225.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Rosser D. S., Schmidt M. C., Berk A. A TATA box implicated in E1A transcriptional activation of a simple adenovirus 2 promoter. Nature. 1987 Apr 2;326(6112):512–515. doi: 10.1038/326512a0. [DOI] [PubMed] [Google Scholar]