Abstract
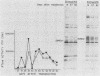
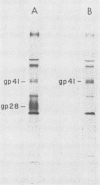
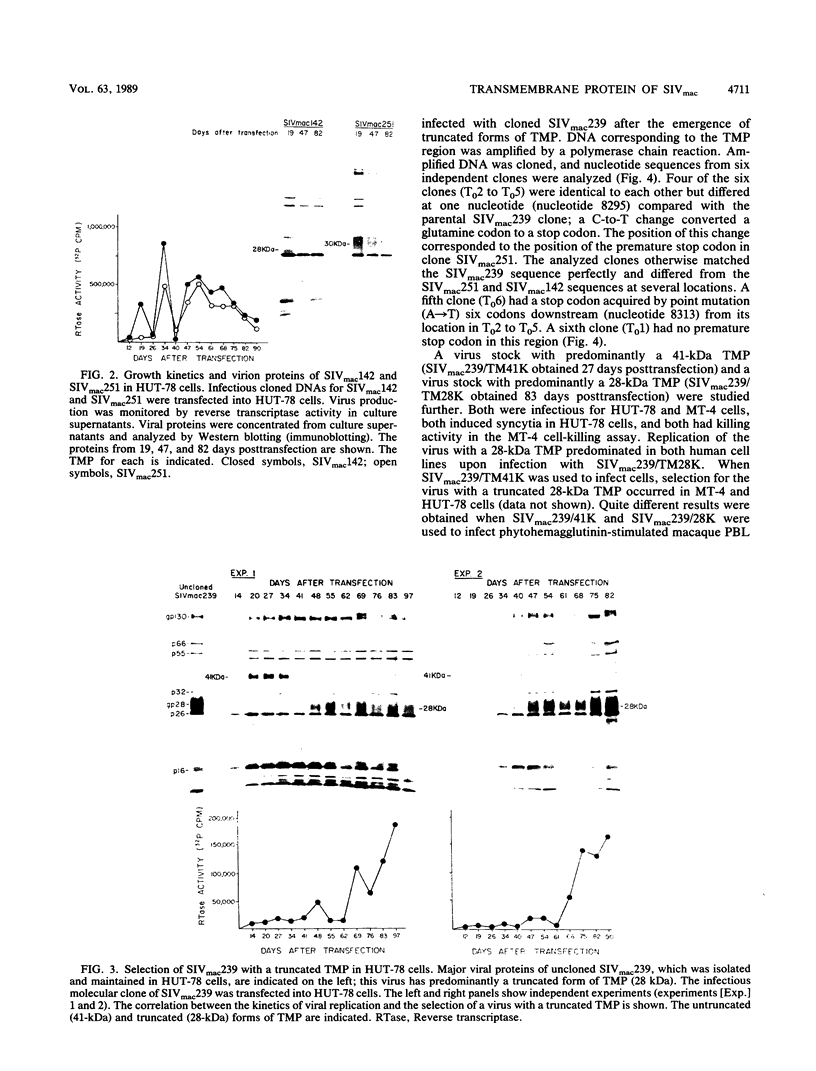
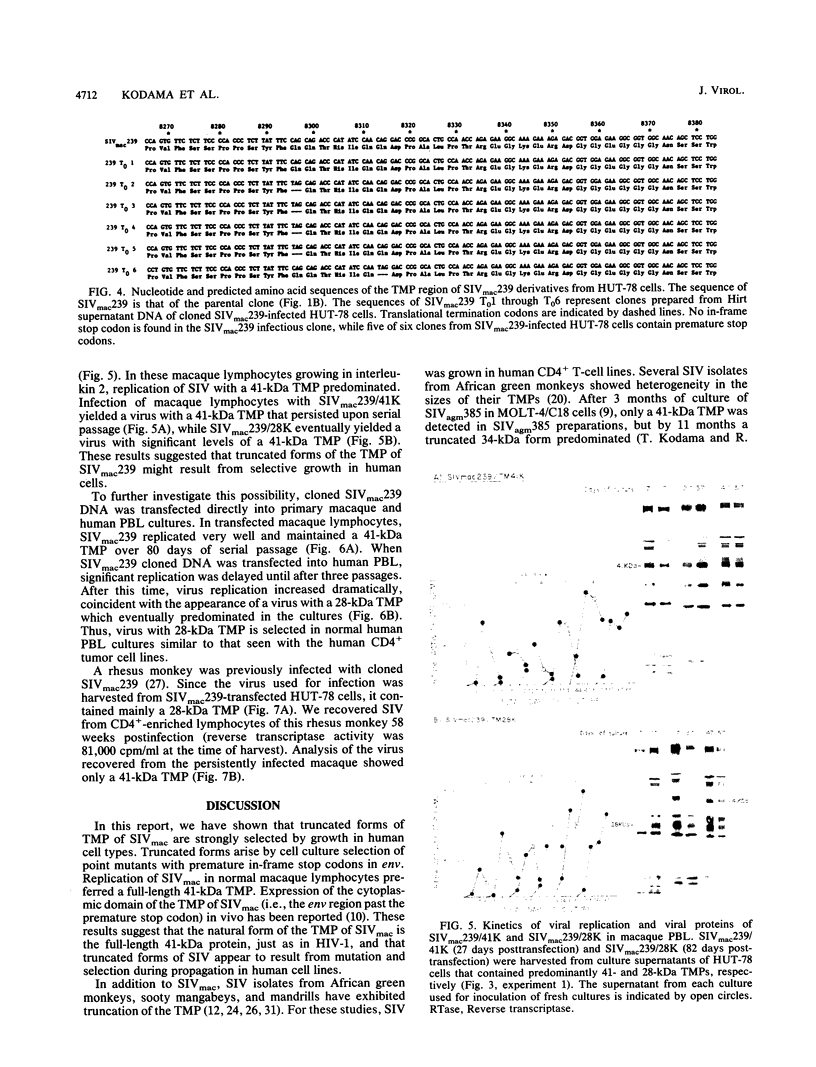
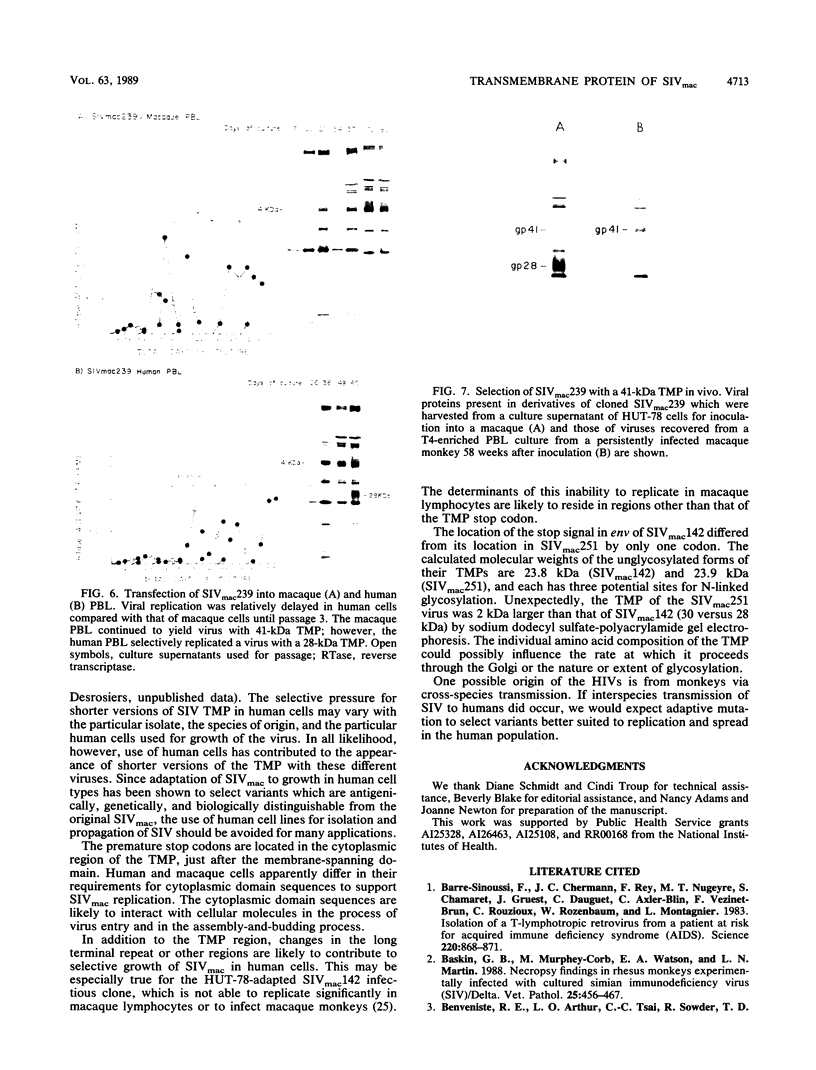
The location of the translational termination codon for the transmembrane protein (TMP) varies in three infectious molecular clones of simian immunodeficiency virus from macaques (SIVmac). The SIVmac251 and SIVmac142 infectious clones have premature stop signals that differ in location by one codon; transfection of these DNAs into human HUT-78 cells yielded virus with a truncated TMP (28 to 30 kilodaltons [kDa]). The SIVmac239 infectious clone does not have a premature stop codon in its TMP-coding region. Transfection of HUT-78 cells with this clone initially yielded virus with a full-length TMP (41 kDa). At 20 to 30 days posttransfection, SIVmac239 virus with a 41-kDa TMP gradually disappeared coincident with the emergence of a virus with a 28-kDa TMP. Virus production dramatically increased in parallel with the emergence of a virus with a 28-kDa TMP. Sequence analysis of viral DNAs from these cultures showed that premature stop codons arising by point mutation were responsible for the change in size of the TMP with time. A similar selective pressure for truncated forms of TMP was observed when the SIVmac239 clone was transfected into human peripheral blood lymphocytes (PBL). In contrast, no such selective pressure was observed in macaque PBL. When the SIVmac239 clone was transfected into macaque PBL and the resultant virus was serially passaged in macaque PBL, the virus replicated very well and maintained a 41-kDa TMP for 80 days in culture. Macaque monkeys were infected with SIVmac239 having a 28-kDa TMP; virus subsequently recovered from T4-enriched lymphocytes of peripheral blood showed only the 41-kDa form of TMP. These results indicate that the natural form of TMP in SIVmac is the full-length 41-kDa TMP, just as in human immunodeficiency virus type 1. Viruses with truncated forms of TMP appear to result from mutation and selection during propagation in unnatural human cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Baskin G. B., Murphey-Corb M., Watson E. A., Martin L. N. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet Pathol. 1988 Nov;25(6):456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Arthur L. O., Tsai C. C., Sowder R., Copeland T. D., Henderson L. E., Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison with HTLV-III/LAV and other lentiviruses. J Virol. 1986 Nov;60(2):483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Hunsmann G., Schmidt D. K., King N. W., Desrosiers R. C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987 Dec;68(Pt 12):3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Schmidt D. K., Silva D. P., Solomon K. R., Hodi F. S., Jr, Ringler D. J., Hunt R. D., King N. W. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer. 1988 Apr 15;41(4):601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Li Y., Naidu Y. M., Durda P. J., Schmidt D. K., Troup C. D., Silva D. P., MacKey J. J., Kestler H. W., 3rd, Sehgal P. K. Simian immunodeficiency virus from African green monkeys. J Virol. 1988 Nov;62(11):4123–4128. doi: 10.1128/jvi.62.11.4123-4128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini G., Kanki P. J., Bosch M. L., Fargnoli K., Wong-Staal F. The simian immunodeficiency virus envelope open reading frame located after the termination codon is expressed in vivo in infected animals. AIDS Res Hum Retroviruses. 1988 Aug;4(4):251–258. doi: 10.1089/aid.1988.4.251. [DOI] [PubMed] [Google Scholar]
- Fukasawa M., Miura T., Hasegawa A., Morikawa S., Tsujimoto H., Miki K., Kitamura T., Hayami M. Sequence of simian immunodeficiency virus from African green monkey, a new member of the HIV/SIV group. Nature. 1988 Jun 2;333(6172):457–461. doi: 10.1038/333457a0. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell J. P., Heath J. L., Hicks D. R., Sporborg C., McGrath C. R., Kalyanaraman V. S. Continuous production of a cytopathic human T-lymphotropic virus in a permissive neoplastic T-cell line. J Clin Microbiol. 1986 Apr;23(4):737–742. doi: 10.1128/jcm.23.4.737-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Kong L. I., Lee S. W., Kumar P., Taylor M. E., Arya S. K., Shaw G. M. Relation of HTLV-4 to simian and human immunodeficiency-associated viruses. Nature. 1987 Nov 12;330(6144):184–186. doi: 10.1038/330184a0. [DOI] [PubMed] [Google Scholar]
- Hirsch V., Riedel N., Mullins J. I. The genome organization of STLV-3 is similar to that of the AIDS virus except for a truncated transmembrane protein. Cell. 1987 May 8;49(3):307–319. doi: 10.1016/0092-8674(87)90283-2. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Chalifoux L. V., Lord C. I., Letvin N. L. Suppression of simian immunodeficiency virus replication in vitro by CD8+ lymphocytes. J Immunol. 1988 Apr 1;140(7):2237–2242. [PubMed] [Google Scholar]
- Kannagi M., Yetz J. M., Letvin N. L. In vitro growth characteristics of simian T-lymphotropic virus type III. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7053–7057. doi: 10.1073/pnas.82.20.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Ohta Y., Masuda T., Ishikawa K., Tsujimoto H., Isahakia M., Hayami M. Production and characterization of monoclonal antibodies specific for the transmembrane protein of simian immunodeficiency virus from the African green monkey. J Virol. 1988 Dec;62(12):4782–4785. doi: 10.1128/jvi.62.12.4782-4785.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Lowenstine L. J., Pedersen N. C., Higgins J., Pallis K. C., Uyeda A., Marx P., Lerche N. W., Munn R. J., Gardner M. B. Seroepidemiologic survey of captive Old-World primates for antibodies to human and simian retroviruses, and isolation of a lentivirus from sooty mangabeys (Cercocebus atys). Int J Cancer. 1986 Oct 15;38(4):563–574. doi: 10.1002/ijc.2910380417. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Masuda T., Tsujimoto H., Ishikawa K., Kodama T., Morikawa S., Nakai M., Honjo S., Hayami M. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int J Cancer. 1988 Jan 15;41(1):115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ringler D. J., Wyand M. S., Walsh D. G., MacKey J. J., Chalifoux L. V., Popovic M., Minassian A. A., Sehgal P. K., Daniel M. D., Desrosiers R. C. Cellular localization of simian immunodeficiency virus in lymphoid tissues. I. Immunohistochemistry and electron microscopy. Am J Pathol. 1989 Feb;134(2):373–383. [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto H., Cooper R. W., Kodama T., Fukasawa M., Miura T., Ohta Y., Ishikawa K., Nakai M., Frost E., Roelants G. E. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J Virol. 1988 Nov;62(11):4044–4050. doi: 10.1128/jvi.62.11.4044-4050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., Joseph B., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Identification of simian immunodeficiency virus SIVMAC env gene products. J Virol. 1989 Mar;63(3):1416–1419. doi: 10.1128/jvi.63.3.1416-1419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagury J. F., Franchini G., Reitz M., Collalti E., Starcich B., Hall L., Fargnoli K., Jagodzinski L., Guo H. G., Laure F. Genetic variability between isolates of human immunodeficiency virus (HIV) type 2 is comparable to the variability among HIV type 1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5941–5945. doi: 10.1073/pnas.85.16.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]