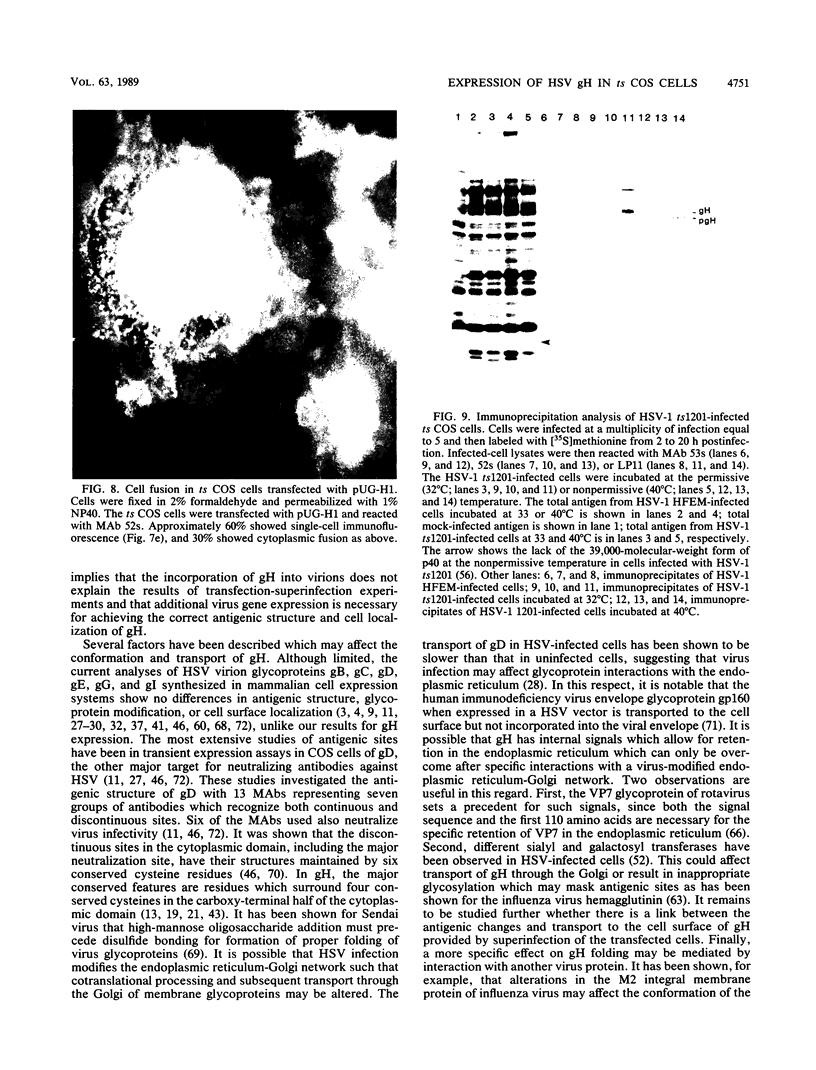
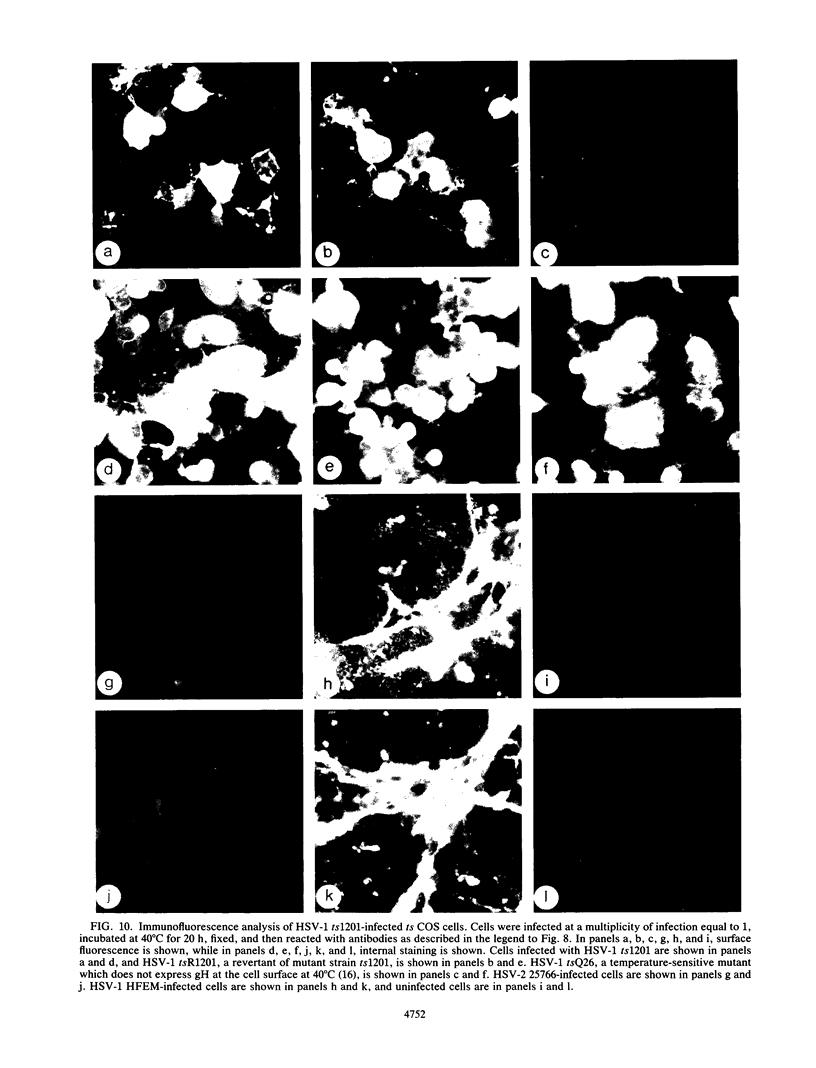
Abstract
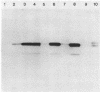
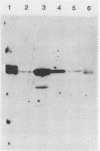
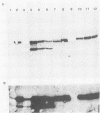
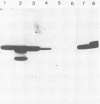
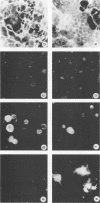
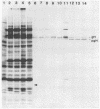
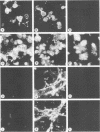
Herpes simplex virus type 1 glycoprotein H (HSV-1 gH) was synthesized in an inducible mammalian cell expression system, and its properties were examined. The gH coding sequence, together with the stable 5' untranslated leader sequence from xenopus beta-globin, was placed under control of the strong promoter from the human cytomegalovirus major immediate-early gene in an amplifiable plasmid which contains the simian virus 40 (SV40) virus origin for replication (ori). This expression vector was transfected into ts COS cells constitutively expressing a temperature-sensitive SV40 T antigen which allows utilization of the SV40 ori at permissive temperatures. The results of transient expression assays at the permissive temperature showed that HSV-1 gH could be synthesized in greater amounts than those produced by a high-multiplicity virus infection. The proteins produced were detected in Western blots (immunoblots) with a HSV-1 gH-specific polyclonal serum raised against a TrpE-gH fusion protein. The transfected gH had an apparent molecular weight of approximately 105,000, intermediate in size to those of the precursor (100,000) and fully processed forms (110,000) of HSV-1 gH from infections. Antigenicity was investigated by reactions with three virus-neutralizing monoclonal antibodies specific for conformational epitopes on gH. Only one of these monoclonal antibodies could immunoprecipitate the synthesized gH. However, equal recognition of the transfected gH was achieved by superinfection with virus. In addition, detectable amounts of gH were not expressed on the cell surface unless the cells were superinfected with virus. Studies with a temperature-sensitive mutant, ts1201, defective in encapsidation showed that the changes in antigenic structure and cell surface expression caused by superinfection with virus were not due simply to incorporation of gH into virions. These results suggest that gH requires additional virus gene products for cell surface localization and formation of an antigenic structure important for its function in mediating infectivity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Longnecker R., Roizman B., Pereira L. Identification, properties, and gene location of a novel glycoprotein specified by herpes simplex virus 1. Virology. 1986 Apr 15;150(1):207–220. doi: 10.1016/0042-6822(86)90280-1. [DOI] [PubMed] [Google Scholar]
- Addison C., Rixon F. J., Palfreyman J. W., O'Hara M., Preston V. G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984 Oct 30;138(2):246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Arsenakis M., Campadelli-Fiume G., Roizman B. Regulation of glycoprotein D synthesis: does alpha 4, the major regulatory protein of herpes simplex virus 1, regulate late genes both positively and negatively? J Virol. 1988 Jan;62(1):148–158. doi: 10.1128/jvi.62.1.148-158.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenakis M., Hubenthal-Voss J., Campadelli-Fiume G., Pereira L., Roizman B. Construction and properties of a cell line constitutively expressing the herpes simplex virus glycoprotein B dependent on functional alpha 4 protein synthesis. J Virol. 1986 Nov;60(2):674–682. doi: 10.1128/jvi.60.2.674-682.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Smith M. H., Hall C. B., Betts R., Hay A. J. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 1988 May;62(5):1508–1512. doi: 10.1128/jvi.62.5.1508-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster E. A., Cranage M. P., McLean C. S., Coombs R. R., Minson A. The use of monoclonal antibodies to differentiate isolates of herpes simplex types 1 and 2 by neutralisation and reverse passive haemagglutination tests. J Med Virol. 1984;13(2):193–202. doi: 10.1002/jmv.1890130209. [DOI] [PubMed] [Google Scholar]
- Buckmaster E. A., Gompels U., Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 X 10(3) molecular weight. Virology. 1984 Dec;139(2):408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C., Sodora D. L., Long D., Levin J. Z., Eisenberg R. J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988 Jun;62(6):1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Smith G. L., Bell S. E., Hart H., Brown C., Bankier A. T., Tomlinson P., Barrell B. G., Minson T. C. Identification and expression of a human cytomegalovirus glycoprotein with homology to the Epstein-Barr virus BXLF2 product, varicella-zoster virus gpIII, and herpes simplex virus type 1 glycoprotein H. J Virol. 1988 Apr;62(4):1416–1422. doi: 10.1128/jvi.62.4.1416-1422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Taylor P. Genetic relations between varicella-zoster virus and Epstein-Barr virus. J Gen Virol. 1987 Apr;68(Pt 4):1067–1079. doi: 10.1099/0022-1317-68-4-1067. [DOI] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of gene organization in the lymphotropic herpesviruses herpesvirus Saimiri and Epstein-Barr virus. J Virol. 1988 Mar;62(3):757–767. doi: 10.1128/jvi.62.3.757-767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Craxton M. A., Honess R. W. Conservation of glycoprotein H (gH) in herpesviruses: nucleotide sequence of the gH gene from herpesvirus saimiri. J Gen Virol. 1988 Nov;69(Pt 11):2819–2829. doi: 10.1099/0022-1317-69-11-2819. [DOI] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Wolstenholme A. J., Skehel J. J., Smith M. H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985 Nov;4(11):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineman T., Gong M., Sample J., Kieff E. Identification of the Epstein-Barr virus gp85 gene. J Virol. 1988 Apr;62(4):1101–1107. doi: 10.1128/jvi.62.4.1101-1107.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Homa F. L., Marlin S. D., Levine M., Glorioso J. Herpes simplex virus type 1 glycoprotein C-negative mutants exhibit multiple phenotypes, including secretion of truncated glycoproteins. J Virol. 1984 Nov;52(2):566–574. doi: 10.1128/jvi.52.2.566-574.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isola V. J., Eisenberg R. J., Siebert G. R., Heilman C. J., Wilcox W. C., Cohen G. H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989 May;63(5):2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987 Jul;61(7):2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Ligas M. W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988 Dec;62(12):4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Smiley J. R. Intracellular transport of herpes simplex virus gD occurs more rapidly in uninfected cells than in infected cells. J Virol. 1985 Jun;54(3):682–689. doi: 10.1128/jvi.54.3.682-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Spear P. G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989 Feb;63(2):819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. M., Davison A. J., Lowe R. S., Riemen M. W., Ellis R. W. Identification and sequence of the gene encoding gpIII, a major glycoprotein of varicella-zoster virus. Virology. 1987 Apr;157(2):526–533. doi: 10.1016/0042-6822(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987 May 1;236(4801):573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986 May;58(2):583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Gualandri R., Negrini M., Albonici L., Milanesi G., Cassai E., Barbanti-Brodano G. Constitutive expression in human cells of herpes simplex virus type 1 glycoprotein B gene cloned in an episomal eukaryotic vector. Virology. 1988 Nov;167(1):284–288. doi: 10.1016/0042-6822(88)90080-3. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N., Hutt-Fletcher L. M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J Virol. 1988 Jul;62(7):2366–2372. doi: 10.1128/jvi.62.7.2366-2372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Muggeridge M. I., Isola V. J., Byrn R. A., Tucker T. J., Minson A. C., Glorioso J. C., Cohen G. H., Eisenberg R. J. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol. 1988 Sep;62(9):3274–3280. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Naujokas M. A., Hassell J. A. Isolation of large T antigen-producing mouse cell lines capable of supporting replication of polyomavirus-plasmid recombinants. Mol Cell Biol. 1984 Nov;4(11):2406–2412. doi: 10.1128/mcb.4.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. A., Reynolds-Kohler C., Smith B. A. Negative and positive regulation by a short segment in the 5'-flanking region of the human cytomegalovirus major immediate-early gene. Mol Cell Biol. 1987 Nov;7(11):4125–4129. doi: 10.1128/mcb.7.11.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A. G., Lee G. T., Sprague R., Parish M. L., Spear P. G. Anti-gD monoclonal antibodies inhibit cell fusion induced by herpes simplex virus type 1. Virology. 1983 Aug;129(1):218–224. doi: 10.1016/0042-6822(83)90409-9. [DOI] [PubMed] [Google Scholar]
- Oba D. E., Hutt-Fletcher L. M. Induction of antibodies to the Epstein-Barr virus glycoprotein gp85 with a synthetic peptide corresponding to a sequence in the BXLF2 open reading frame. J Virol. 1988 Apr;62(4):1108–1114. doi: 10.1128/jvi.62.4.1108-1114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S., Khanna B., Lycke E. Altered kinetic properties of sialyl and galactosyl transferases associated with herpes simplex virus infection of GMK and BHK cells. J Gen Virol. 1980 Mar;47(1):1–9. doi: 10.1099/0022-1317-47-1-1. [DOI] [PubMed] [Google Scholar]
- Pachl C., Probert W. S., Hermsen K. M., Masiarz F. R., Rasmussen L., Merigan T. C., Spaete R. R. The human cytomegalovirus strain Towne glycoprotein H gene encodes glycoprotein p86. Virology. 1989 Apr;169(2):418–426. doi: 10.1016/0042-6822(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Para M. F., Parish M. L., Noble A. G., Spear P. G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985 Aug;55(2):483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen L. E., Nelson R. M., Kelsall D. C., Merigan T. C. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc Natl Acad Sci U S A. 1984 Feb;81(3):876–880. doi: 10.1073/pnas.81.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Buckmaster A., Bell S., Hodgman C., Minson A. C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J Virol. 1986 Feb;57(2):647–655. doi: 10.1128/jvi.57.2.647-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C., Clark S. G., Tjian R. A mammalian host-vector system that regulates expression and amplification of transfected genes by temperature induction. Science. 1985 Jan 4;227(4682):23–28. doi: 10.1126/science.2981116. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Seidel-Dugan C., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Cohen G. H., Eisenberg R. J. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J Virol. 1988 Nov;62(11):4027–4036. doi: 10.1128/jvi.62.11.4027-4036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelbourn S. L., Sissons J. G., Sinclair J. H. Expression of oncogenic ras in human teratocarcinoma cells induces partial differentiation and permissiveness for human cytomegalovirus infection. J Gen Virol. 1989 Feb;70(Pt 2):367–374. doi: 10.1099/0022-1317-70-2-367. [DOI] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Stevens D. J., Daniels R. S., Douglas A. R., Knossow M., Wilson I. A., Wiley D. C. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirzaker S. C., Both G. W. The signal peptide of the rotavirus glycoprotein VP7 is essential for its retention in the ER as an integral membrane protein. Cell. 1989 Mar 10;56(5):741–747. doi: 10.1016/0092-8674(89)90677-6. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Schuster T., Klein R., Hopkins R. F., 3rd, Witmer T., Neubauer R. H., Rabin H. Production and characterization of monoclonal antibodies against the Epstein-Barr virus membrane antigen. J Virol. 1982 Jan;41(1):258–264. doi: 10.1128/jvi.41.1.258-264.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. K., Courtney R. J. Inducible expression of herpes simplex virus type 2 glycoprotein gene gG-2 in a mammalian cell line. J Virol. 1988 Oct;62(10):3668–3674. doi: 10.1128/jvi.62.10.3668-3674.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Mottet G., Kolakofsky D., Roux L. Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J Virol. 1989 Feb;63(2):892–900. doi: 10.1128/jvi.63.2.892-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]
- Whealy M. E., Baumeister K., Robbins A. K., Enquist L. W. A herpesvirus vector for expression of glycosylated membrane antigens: fusion proteins of pseudorabies virus gIII and human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1988 Nov;62(11):4185–4194. doi: 10.1128/jvi.62.11.4185-4194.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox W. C., Long D., Sodora D. L., Eisenberg R. J., Cohen G. H. The contribution of cysteine residues to antigenicity and extent of processing of herpes simplex virus type 1 glycoprotein D. J Virol. 1988 Jun;62(6):1941–1947. doi: 10.1128/jvi.62.6.1941-1947.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]