Abstract
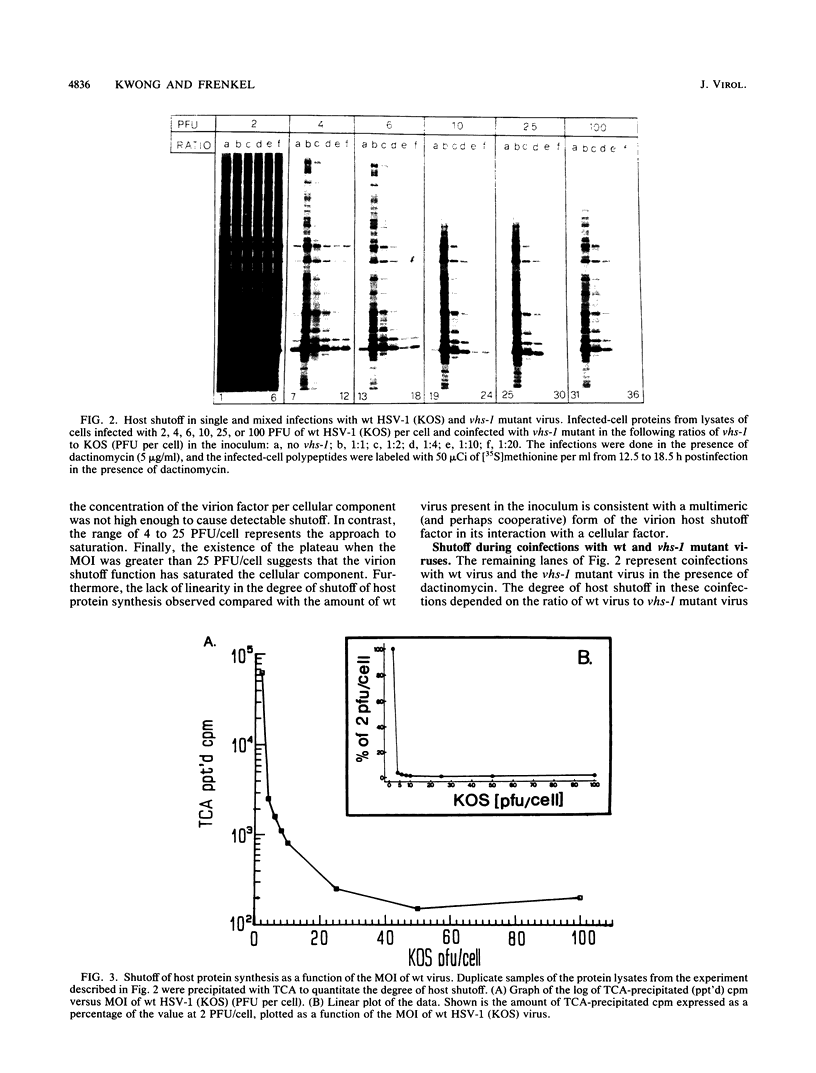
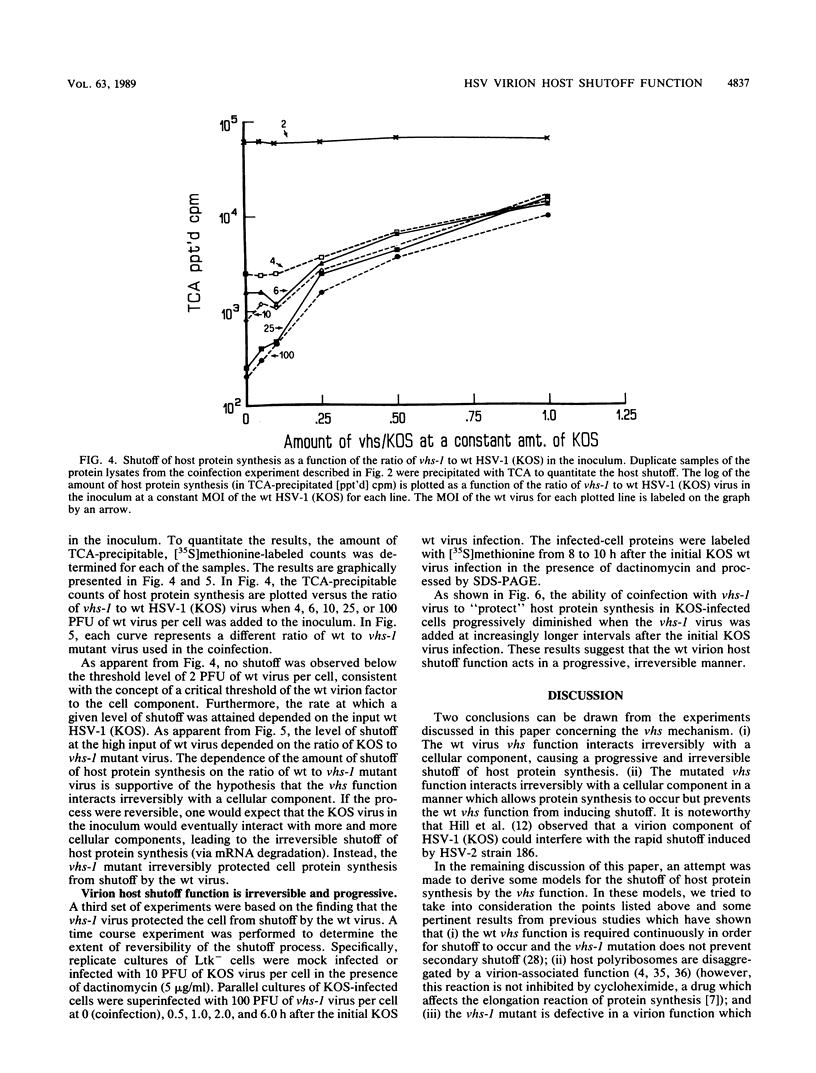
The virion host shutoff (vhs) function of herpes simplex virus (HSV) limits the expression of genes in the infected cells by destabilizing both host and viral mRNAs. vhs function mutants have been isolated which are defective in their ability to degrade host mRNA. Furthermore, the half-life of viral mRNAs is significantly longer in cells infected with the vhs-1 mutant virus than in cells infected with the wild-type (wt) virus. Recent data have shown that the vhs-1 mutation resides within the open reading frame UL41. We have analyzed the shutoff of host protein synthesis in cells infected with a mixture of the wt HSV-1 (KOS) and the vhs-1 mutant virus. The results of these experiments revealed that (i) the wt virus shutoff activity requires a threshold level of input virions per cell and (ii) the mutant vhs-1 virus protein can irreversibly block the wt virus shutoff activity. These results are consistent with a stoichiometric model in which the wt vhs protein interacts with a cellular factor which controls the half-life of cell mRNA. This wt virus interaction results in the destabilization of both host and viral mRNAs. In contrast, the mutant vhs function interacts with the cellular factor irreversibly, resulting in the increased half-life of both host and viral mRNAs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastow K. F., Bouchard J., Ren X. J., Cheng Y. C. Synthesis of dihydrofolate reductase and metabolism of related RNA in a methotrexate resistant human cell line infected with herpes simplex virus type 2. Virology. 1986 Mar;149(2):199–207. doi: 10.1016/0042-6822(86)90121-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L., Clark J. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J Gen Virol. 1982 Jul;61(Pt 50):121–125. doi: 10.1099/0022-1317-61-1-121. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978 Oct;41(1):37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- Fenwick M., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XI. Apparent clustering of functions effecting rapid inhibition of host DNA and protein synthesis. J Virol. 1979 Feb;29(2):825–827. doi: 10.1128/jvi.29.2.825-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frink R. J., Anderson K. P., Wagner E. K. Herpes simplex virus type 1 HindIII fragment L encodes spliced and complementary mRNA species. J Virol. 1981 Aug;39(2):559–572. doi: 10.1128/jvi.39.2.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. Transformation of rodent cells by a cloned DNA fragment of herpes simplex virus type 2. J Virol. 1981 May;38(2):749–760. doi: 10.1128/jvi.38.2.749-760.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A. L., Sandri-Goldin R. M., Levine M., Glorioso J. C. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol. 1981 Apr;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Sadler J. R., Betz J. L. Virion component of herpes simplex virus type 1 KOS interferes with early shutoff of host protein synthesis induced by herpes simplex virus type 2 186. J Virol. 1985 Oct;56(1):312–316. doi: 10.1128/jvi.56.1.312-316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C. Inhibition of host protein synthesis and degradation of cellular mRNAs during infection by influenza and herpes simplex virus. Mol Cell Biol. 1982 Dec;2(12):1644–1648. doi: 10.1128/mcb.2.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. D., Kruper J. A., Frenkel N. Herpes simplex virus virion host shutoff function. J Virol. 1988 Mar;62(3):912–921. doi: 10.1128/jvi.62.3.912-921.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker H., Frenkel N., Halliburton I. Structure and expression of class II defective herpes simplex virus genomes encoding infected cell polypeptide number 8. J Virol. 1982 Aug;43(2):574–593. doi: 10.1128/jvi.43.2.574-593.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayman B. A., Nishioka Y. Differential stability of host mRNAs in Friend erythroleukemia cells infected with herpes simplex virus type 1. J Virol. 1985 Jan;53(1):1–6. doi: 10.1128/jvi.53.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H., Maxwell I. H., Pizer L. I. Herpesvirus infection alters the steady-state levels of cellular polyadenylated RNA in polyoma virus-transformed BHK cells. J Virol. 1982 Jun;42(3):1131–1134. doi: 10.1128/jvi.42.3.1131-1134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Alterations in the protein synthetic apparatus of Friend erythroleukemia cells infected with vesicular stomatitis virus or herpes simplex virus. J Virol. 1978 Jan;25(1):422–426. doi: 10.1128/jvi.25.1.422-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroskar A. A., Read G. S. A mutant of herpes simplex virus type 1 exhibits increased stability of immediate-early (alpha) mRNAs. J Virol. 1987 Feb;61(2):604–606. doi: 10.1128/jvi.61.2.604-606.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroskar A. A., Read G. S. Control of mRNA stability by the virion host shutoff function of herpes simplex virus. J Virol. 1989 May;63(5):1897–1906. doi: 10.1128/jvi.63.5.1897-1906.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Beard P. The effect of herpes virus infection on mRNA in polyoma virus transformed cells. Virology. 1976 Dec;75(2):477–480. doi: 10.1016/0042-6822(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schek N., Bachenheimer S. L. Degradation of cellular mRNAs induced by a virion-associated factor during herpes simplex virus infection of Vero cells. J Virol. 1985 Sep;55(3):601–610. doi: 10.1128/jvi.55.3.601-610.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. R., Fong B. S., Leung W. C. Construction of a double-jointed herpes simplex viral DNA molecule: inverted repeats are required for segment inversion, and direct repeats promote deletions. Virology. 1981 Aug;113(1):345–362. doi: 10.1016/0042-6822(81)90161-6. [DOI] [PubMed] [Google Scholar]
- Stenberg R. M., Pizer L. I. Herpes simplex virus-induced changes in cellular and adenovirus RNA metabolism in an adenovirus type 5-transformed human cell line. J Virol. 1982 May;42(2):474–487. doi: 10.1128/jvi.42.2.474-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer J. R., Holland L. E., Swanstrom R. I., Pivo K., Wagner E. K. Quantitation of herpes simplex virus type 1 RNA in infected HeLa cells. J Virol. 1977 Mar;21(3):889–901. doi: 10.1128/jvi.21.3.889-901.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom T., Frenkel N. Effects of herpes simplex virus on mRNA stability. J Virol. 1987 Jul;61(7):2198–2207. doi: 10.1128/jvi.61.7.2198-2207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]