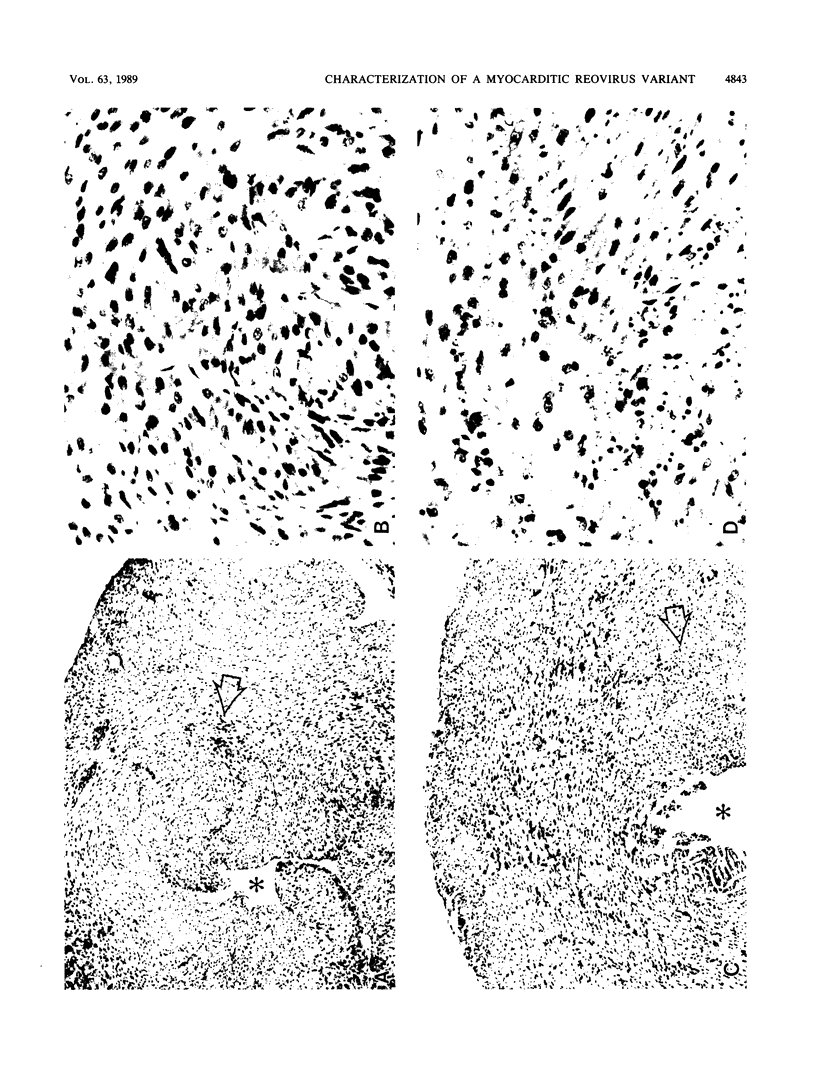
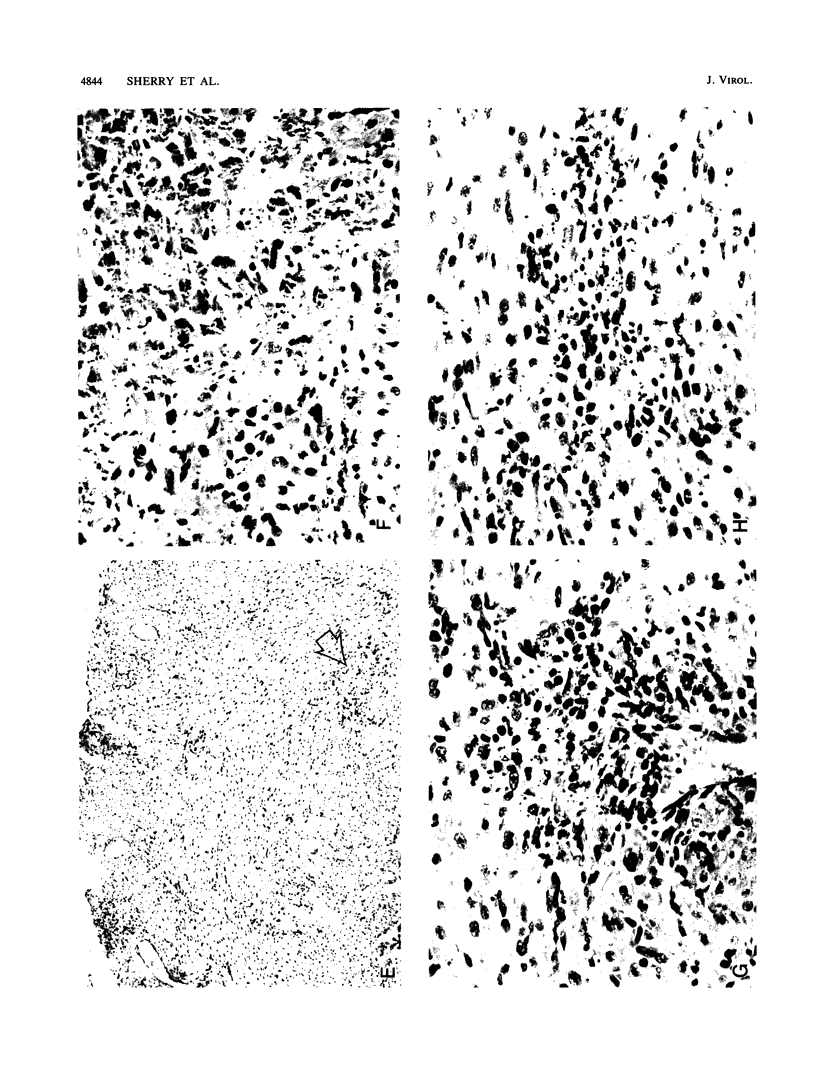
Abstract
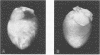
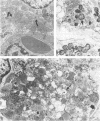
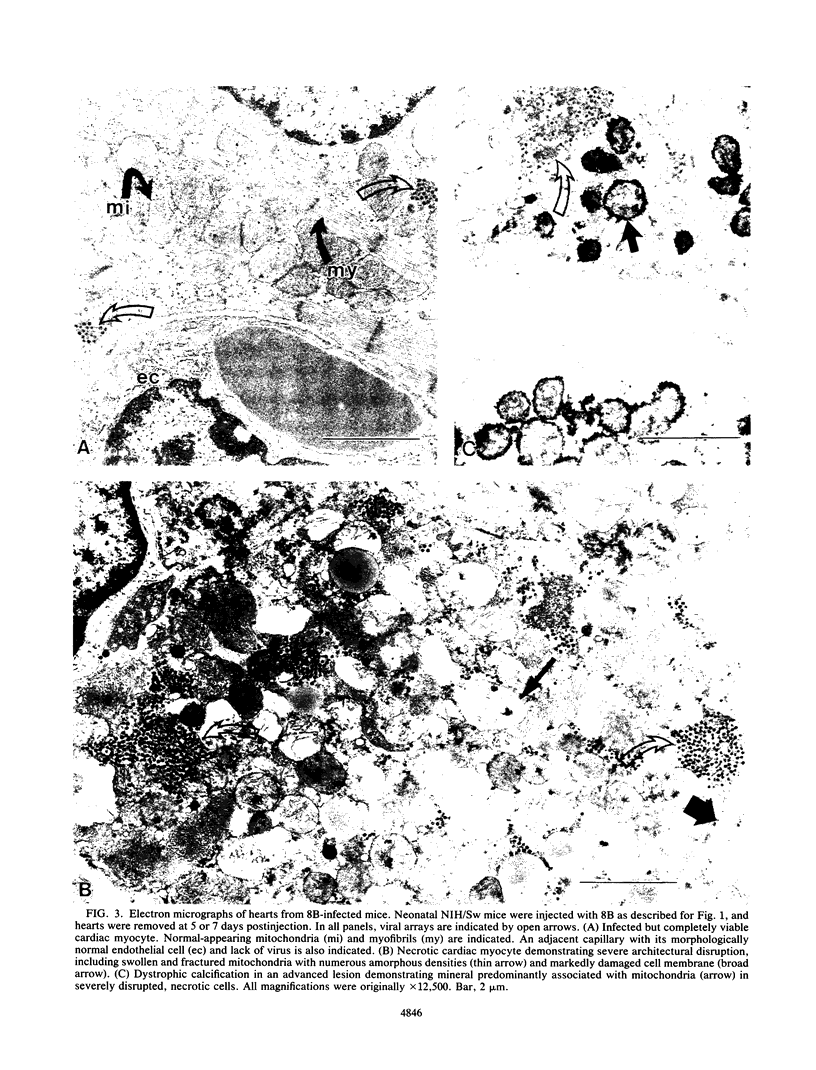
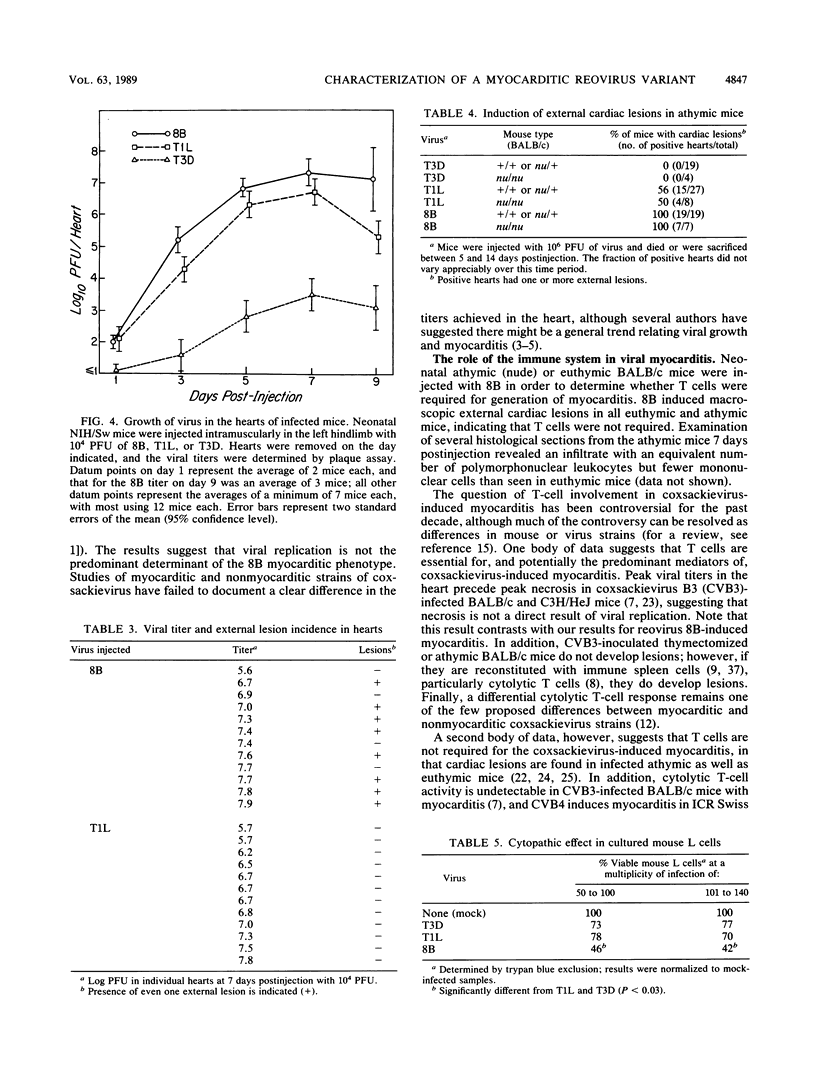
A reovirus variant, 8B, was isolated from a neonatal mouse which had been inoculated with a mixture of two reovirus strains: type 1 Lang (T1L) and type 3 Dearing (T3D) (E. A. Wenske, S.J. Chanock, L. Krata, and B. N. Fields, J. Virol. 56:613-616, 1985). 8B is a reassortant containing eight gene segments derived from the T1L parent and two gene segments derived from the T3D parent. Upon infection of neonatal mice, 8B produced a generalized infection characteristic of many reoviruses, but it also efficiently induced numerous macroscopic external cardiac lesions, unlike either of its parents. Microscopic examination of hearts from infected mice revealed myocarditis with necrotic myocytes and both polymorphonuclear and mononuclear cellular infiltration. Electron microscopy revealed viral arrays in necrotic myocytes and dystrophic calcification accompanying late lesions. Determination of viral titers in hearts from T1L-, T3D-, or 8B-infected mice indicated that growth was not the primary determinant of myocardial necrosis. Results from inoculations of athymic mice demonstrated that T cells were not a requirement for the 8B-induced myocarditis. Finally, 8B was more cytopathic than either of the parent viruses in cultured mouse L cells. Together, the data suggest that 8B-induced myocardial necrosis is due to a direct effect of reovirus on myocytes. Reovirus thus provides a useful model for the study of viral myocarditis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aretz H. T., Billingham M. E., Edwards W. D., Factor S. M., Fallon J. T., Fenoglio J. J., Jr, Olsen E. G., Schoen F. J. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987 Jan;1(1):3–14. [PubMed] [Google Scholar]
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Cao Y., Schnurr D. P., Schmidt N. J. Differing cardiotropic and myocarditic properties of group B type 4 coxsackievirus strains. Arch Virol. 1984;80(2-3):119–130. doi: 10.1007/BF01310653. [DOI] [PubMed] [Google Scholar]
- Cao Y., Schnurr D. P., Schmidt N. J. Monoclonal antibodies for study of antigenic variation in coxsackievirus type B4: association of antigenic determinants with myocarditic properties of the virus. J Gen Virol. 1984 May;65(Pt 5):925–932. doi: 10.1099/0022-1317-65-5-925. [DOI] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., LaBadie D. R., Paque R. E., Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3(3):207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- Grun J. B., Schultz M., Finkelstein S. D., Crowell R. L., Landau B. J. Pathogenesis of acute myocardial necrosis in inbred mice infected with coxsackievirus B3. Microb Pathog. 1988 Jun;4(6):417–430. doi: 10.1016/0882-4010(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Guthrie M., Lodge P. A., Huber S. A. Cardiac injury in myocarditis induced by Coxsackievirus group B, type 3 in Balb/c mice is mediated by Lyt 2 + cytolytic lymphocytes. Cell Immunol. 1984 Oct 15;88(2):558–567. doi: 10.1016/0008-8749(84)90188-6. [DOI] [PubMed] [Google Scholar]
- Göller T., Galle J., Eggers H. J., Bültmann B. Experimental reovirus myocarditis in newborn mice. Electron microscopic observations. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;50(4):373–386. doi: 10.1007/BF02889915. [DOI] [PubMed] [Google Scholar]
- HASSAN S. A., RABIN E. R., MELNICK J. L. REOVIRUS MYOCARDITIS IN MICE: AN ELECTRON MICROSCOPIC, IMMUNOFLUORESCENT, AND VIRUS ASSAY STUDY. Exp Mol Pathol. 1965 Feb;76:66–80. doi: 10.1016/0014-4800(65)90024-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto I., Tatsumi M., Nakagawa M. The role of T lymphocytes in the pathogenesis of Coxsackie virus B3 heart disease. Br J Exp Pathol. 1983 Oct;64(5):497–504. [PMC free article] [PubMed] [Google Scholar]
- Huber S. A., Job L. P., Auld K. R., Woodruff J. F. Sex-related differences in the rapid production of cytotoxic spleen cells active against uninfected myofibers during Coxsackievirus B-3 infection. J Immunol. 1981 Apr;126(4):1336–1340. [PubMed] [Google Scholar]
- Huber S. A., Job L. P. Differences in cytolytic T cell response of BALB/c mice infected with myocarditic and non-myocarditic strains of coxsackievirus group B, type 3. Infect Immun. 1983 Mar;39(3):1419–1427. doi: 10.1128/iai.39.3.1419-1427.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatib R., Khatib G., Chason J. L., Lerner A. M. Alterations in coxsackievirus B4 heart muscle disease in ICR Swiss mice by anti-thymocyte serum. J Gen Virol. 1983 Jan;64(Pt 1):231–236. doi: 10.1099/0022-1317-64-1-231. [DOI] [PubMed] [Google Scholar]
- Khatib R., Probert A., Reyes M. P., Khatib G., Chason J. L. Mouse strain-related variation as a factor in the pathogenesis of coxsackievirus B3 murine myocarditis. J Gen Virol. 1987 Dec;68(Pt 12):2981–2988. doi: 10.1099/0022-1317-68-12-2981. [DOI] [PubMed] [Google Scholar]
- Marboe C. C., Fenoglio J. J., Jr Biopsy diagnosis of myocarditis. Cardiovasc Clin. 1988;18(2):137–154. [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- Neu N., Rose N. R., Beisel K. W., Herskowitz A., Gurri-Glass G., Craig S. W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., O'Connell J. B., Roeges L. M., Major E. O., Gunnar R. M. Coxsackie B3 myocarditis in athymic mice. Proc Soc Exp Biol Med. 1981 Jan;166(1):80–91. doi: 10.3181/00379727-166-41028. [DOI] [PubMed] [Google Scholar]
- Roesing T. G., Landau B. J., Crowell R. L. Limited persistence of viral antigen in coxsackievirus B3 induced heart disease in mice. Proc Soc Exp Biol Med. 1979 Mar;160(3):382–386. doi: 10.3181/00379727-160-40455. [DOI] [PubMed] [Google Scholar]
- Schnurr D. P., Cao Y., Schmidt N. J. Coxsackievirus B3 persistence and myocarditis in N:NIH(S) II nu/nu and +/nu mice. J Gen Virol. 1984 Jul;65(Pt 7):1197–1201. doi: 10.1099/0022-1317-65-7-1197. [DOI] [PubMed] [Google Scholar]
- Schnurr D. P., Schmidt N. J. Coxsackievirus B3 persistence and myocarditis in NFR nu/nu and +/nu mice. Med Microbiol Immunol. 1984;173(1):1–7. doi: 10.1007/BF02123563. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular DNA synthesis: role of the S1 gene. J Virol. 1981 Apr;38(1):389–392. doi: 10.1128/jvi.38.1.389-392.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular RNA and protein synthesis: role of the S4 gene. Virology. 1982 Oct 30;122(2):381–391. doi: 10.1016/0042-6822(82)90237-9. [DOI] [PubMed] [Google Scholar]
- Sherry B., Fields B. N. The reovirus M1 gene, encoding a viral core protein, is associated with the myocarditic phenotype of a reovirus variant. J Virol. 1989 Nov;63(11):4850–4856. doi: 10.1128/jvi.63.11.4850-4856.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Inglis S. C. The mutation rate and variability of eukaryotic viruses: an analytical review. J Gen Virol. 1987 Nov;68(Pt 11):2729–2740. doi: 10.1099/0022-1317-68-11-2729. [DOI] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTERS M. N., LEAK P. J., JOSKE R. A., STANLEY N. F., PERRET D. H. MURINE INFECTION WITH REOVIRUS. 3. PATHOLOGY OF INFECTION WITH TYPES 1 AND 2. Br J Exp Pathol. 1965 Apr;46:200–212. [PMC free article] [PubMed] [Google Scholar]
- Wenske E. A., Chanock S. J., Krata L., Fields B. N. Genetic reassortment of mammalian reoviruses in mice. J Virol. 1985 Nov;56(2):613–616. doi: 10.1128/jvi.56.2.613-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- Woodruff J. F., Woodruff J. J. Involvement of T lymphocytes in the pathogenesis of coxsackie virus B3 heart disease. J Immunol. 1974 Dec;113(6):1726–1734. [PubMed] [Google Scholar]