Abstract
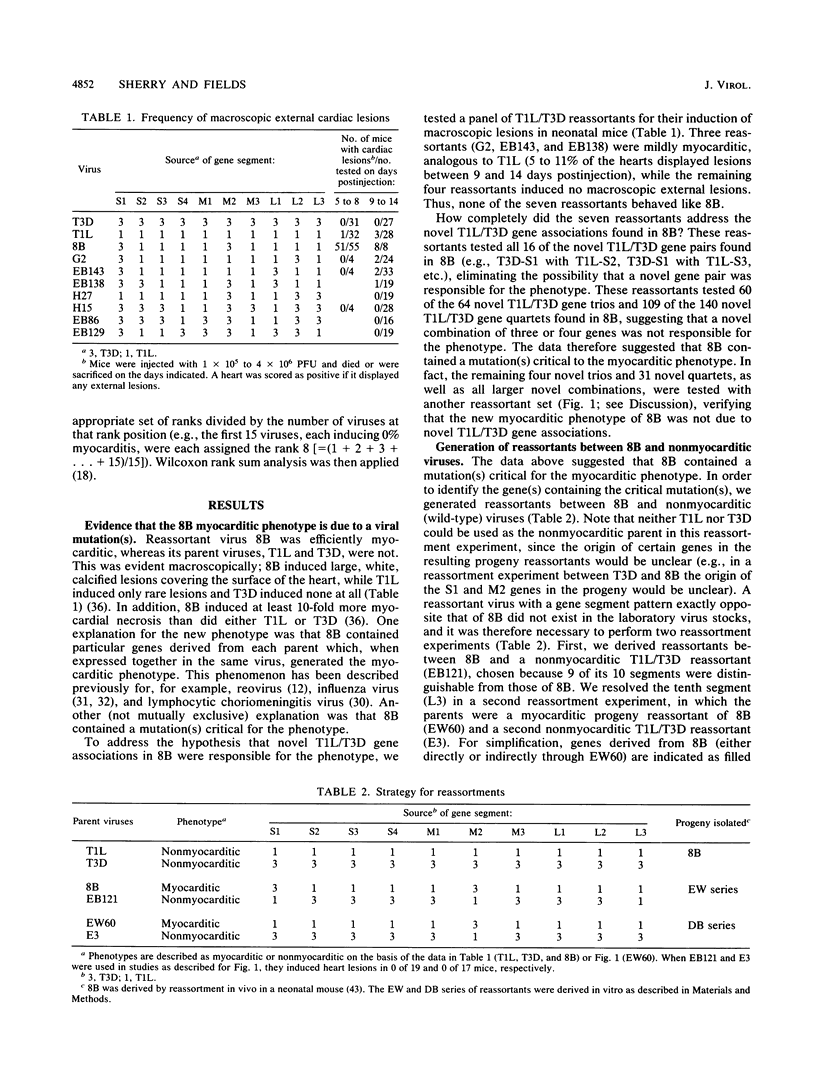
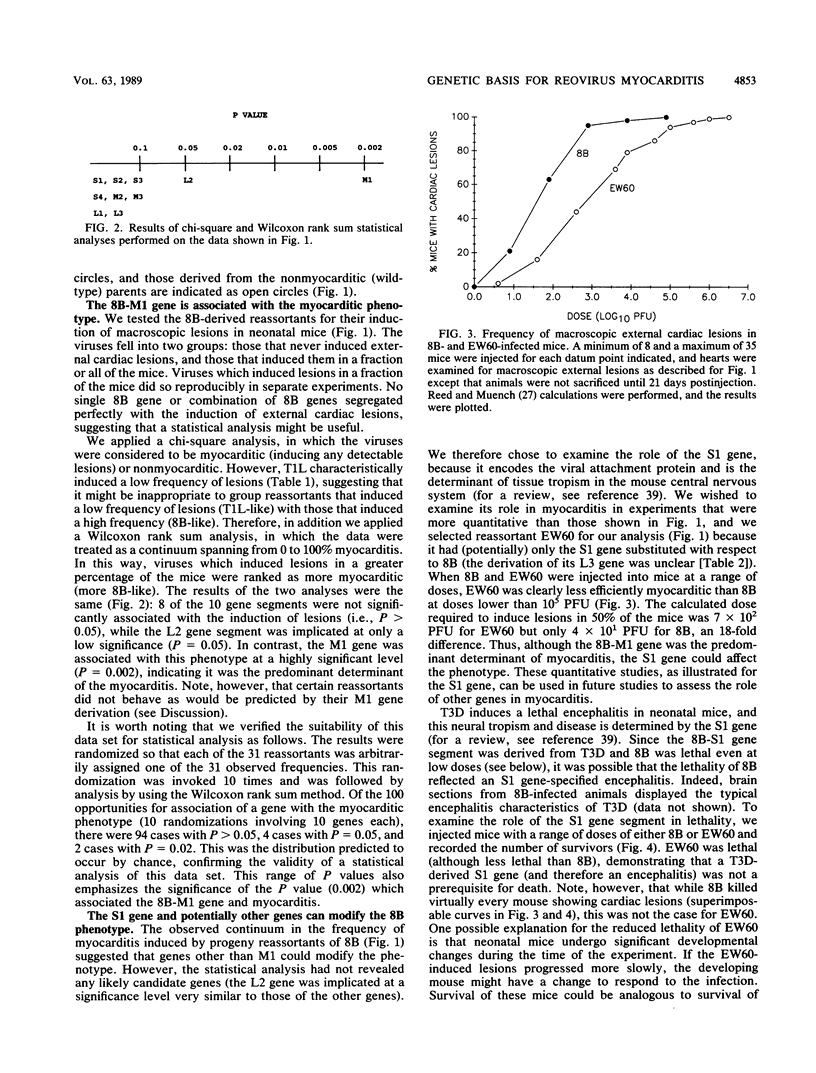
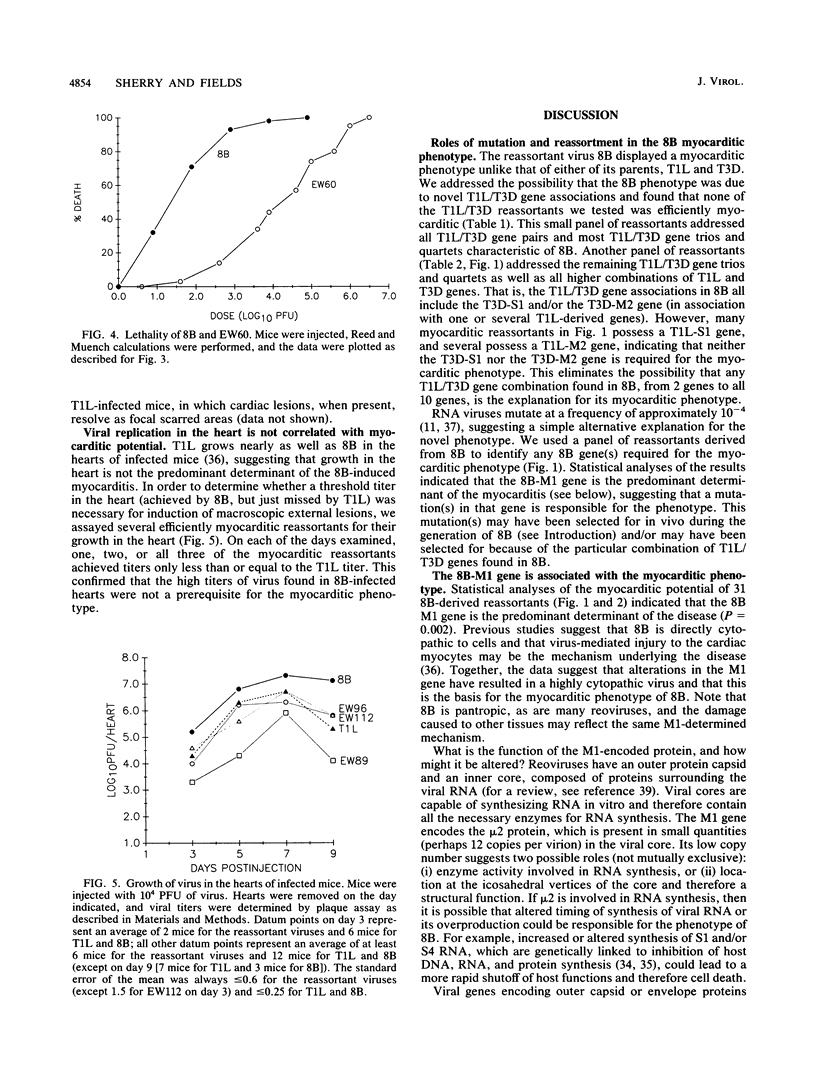
Reoviruses contain a genome composed of 10 double-stranded RNA gene segments. A reovirus reassortant, 8B, derived from type 1 Lang (T1L) and type 3 Dearing (T3D), displayed a phenotype unlike that of either of its parents in that it efficiently induced numerous macroscopic external cardiac lesions in neonatal mice (B. Sherry, F. J. Schoen, E. Wenske, and B. N. Fields, J. Virol. 63:4840-4849, 1989). A panel of T1L/T3D reassortants and a panel of reassortants derived from 8B were used to determine whether novel T1L/T3D gene associations in 8B were responsible for its myocarditic phenotype. The results eliminated the possibility that any T1L/T3D gene combination found in 8B, from 2 genes to all 10 genes, was the explanation for its induction of cardiac lesions. This suggested that a mutation(s) in an 8B gene(s) might be responsible for induction of the myocarditis. Statistical analysis of experiments with 31 reassortants derived from 8B revealed a highly significant association (P = 0.002) of the 8B M1 gene with induction of cardiac lesions. The reovirus M1 gene encodes a viral core protein of unknown function, although evidence suggests a potential role in core structure and/or viral RNA synthesis. This represents the first report of the association of a viral gene with induction of myocarditis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodkin D. K., Fields B. N. Growth and survival of reovirus in intestinal tissue: role of the L2 and S1 genes. J Virol. 1989 Mar;63(3):1188–1193. doi: 10.1128/jvi.63.3.1188-1193.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F. X., Orlich M., Klenk H. D., Rott R. The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology. 1979 May;95(1):197–207. doi: 10.1016/0042-6822(79)90414-8. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Dalziel R. G., Lampert P. W., Talbot P. J., Buchmeier M. J. Site-specific alteration of murine hepatitis virus type 4 peplomer glycoprotein E2 results in reduced neurovirulence. J Virol. 1986 Aug;59(2):463–471. doi: 10.1128/jvi.59.2.463-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wiktor T. J., Trojanowski J. Q., Macfarlan R. I., Wunner W. H., Torres-Anjel M. J., Koprowski H. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J Virol. 1985 Oct;56(1):12–18. doi: 10.1128/jvi.56.1.12-18.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Genetic studies on the mechanism of chemical and physical inactivation of reovirus. J Gen Virol. 1982 Nov;63(Pt 1):149–159. doi: 10.1099/0022-1317-63-1-149. [DOI] [PubMed] [Google Scholar]
- Evans D. M., Dunn G., Minor P. D., Schild G. C., Cann A. J., Stanway G., Almond J. W., Currey K., Maizel J. V., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985 Apr 11;314(6011):548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., el-Zaatari F. A., Stohlman S. A., Weiner L. P. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J Virol. 1986 Jun;58(3):869–875. doi: 10.1128/jvi.58.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy D. B., Rubin D. H., Fields B. N. Molecular basis of reovirus neurovirulence: role of the M2 gene in avirulence. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1298–1302. doi: 10.1073/pnas.79.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keroack M., Fields B. N. Viral shedding and transmission between hosts determined by reovirus L2 gene. Science. 1986 Jun 27;232(4758):1635–1638. doi: 10.1126/science.3012780. [DOI] [PubMed] [Google Scholar]
- Khatib R., Probert A., Reyes M. P., Khatib G., Chason J. L. Mouse strain-related variation as a factor in the pathogenesis of coxsackievirus B3 murine myocarditis. J Gen Virol. 1987 Dec;68(Pt 12):2981–2988. doi: 10.1099/0022-1317-68-12-2981. [DOI] [PubMed] [Google Scholar]
- La Monica N., Almond J. W., Racaniello V. R. A mouse model for poliovirus neurovirulence identifies mutations that attenuate the virus for humans. J Virol. 1987 Sep;61(9):2917–2920. doi: 10.1128/jvi.61.9.2917-2920.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lustig S., Jackson A. C., Hahn C. S., Griffin D. E., Strauss E. G., Strauss J. H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988 Jul;62(7):2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Bradley J., Yang X. F., Wimmer E., Moss E. G., Racaniello V. R. Poliovirus host range is determined by a short amino acid sequence in neutralization antigenic site I. Science. 1988 Jul 8;241(4862):213–215. doi: 10.1126/science.2838906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo J. M., Davis N. L., Rice C. M., Huang H. V., Johnston R. E. Molecular analysis of Sindbis virus pathogenesis in neonatal mice by using virus recombinants constructed in vitro. J Virol. 1988 Jun;62(6):2124–2133. doi: 10.1128/jvi.62.6.2124-2133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehaud C., Coulon P., LaFay F., Thiers C., Flamand A. Antigenic site II of the rabies virus glycoprotein: structure and role in viral virulence. J Virol. 1988 Jan;62(1):1–7. doi: 10.1128/jvi.62.1.1-7.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Cross R. K., Fields B. N. Genome RNAs and polypeptides of reovirus serotypes 1, 2, and 3. J Virol. 1977 Jun;22(3):726–733. doi: 10.1128/jvi.22.3.726-733.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig R. F., Fields B. N. Revertants of temperature-sensitive mutants of reovirus: evidence for frequent extragenic suppression. Virology. 1979 Jan 15;92(1):155–167. doi: 10.1016/0042-6822(79)90221-6. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P. J., Buchmeier M. J., Oldstone M. B. Genetic mapping of lymphocytic choriomeningitis virus pathogenicity: virulence in guinea pigs is associated with the L RNA segment. J Virol. 1985 Sep;55(3):704–709. doi: 10.1128/jvi.55.3.704-709.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P., Oldstone M. B. Perturbation of differentiated functions during viral infection in vivo. II. Viral reassortants map growth hormone defect to the S RNA of the lymphocytic choriomeningitis virus genome. Virology. 1985 Apr 15;142(1):175–182. doi: 10.1016/0042-6822(85)90431-3. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Oldstone M. B. Genetic reassortants of lymphocytic choriomeningitis virus: unexpected disease and mechanism of pathogenesis. J Virol. 1986 Aug;59(2):363–368. doi: 10.1128/jvi.59.2.363-368.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Orlich M., Scholtissek C. Correlation of pathogenicity and gene constellation of influenza A viruses. III. Non-pathogenic recombinants derived from highly pathogenic parent strains. J Gen Virol. 1979 Aug;44(2):471–477. doi: 10.1099/0022-1317-44-2-471. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Vallbracht A., Flehmig B., Rott R. Correlation of pathogenicity and gene constellation of influenza A viruses. II. Highly neurovirulent recombinants derived from non-neurovirulent or weakly neurovirulent parent virus strains. Virology. 1979 Jun;95(2):492–500. doi: 10.1016/0042-6822(79)90503-8. [DOI] [PubMed] [Google Scholar]
- Seif I., Coulon P., Rollin P. E., Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985 Mar;53(3):926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular DNA synthesis: role of the S1 gene. J Virol. 1981 Apr;38(1):389–392. doi: 10.1128/jvi.38.1.389-392.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Fields B. N. Reovirus inhibition of cellular RNA and protein synthesis: role of the S4 gene. Virology. 1982 Oct 30;122(2):381–391. doi: 10.1016/0042-6822(82)90237-9. [DOI] [PubMed] [Google Scholar]
- Sherry B., Schoen F. J., Wenske E., Fields B. N. Derivation and characterization of an efficiently myocarditic reovirus variant. J Virol. 1989 Nov;63(11):4840–4849. doi: 10.1128/jvi.63.11.4840-4849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Inglis S. C. The mutation rate and variability of eukaryotic viruses: an analytical review. J Gen Virol. 1987 Nov;68(Pt 11):2729–2740. doi: 10.1099/0022-1317-68-11-2729. [DOI] [PubMed] [Google Scholar]
- Spriggs D. R., Bronson R. T., Fields B. N. Hemagglutinin variants of reovirus type 3 have altered central nervous system tropism. Science. 1983 Apr 29;220(4596):505–507. doi: 10.1126/science.6301010. [DOI] [PubMed] [Google Scholar]
- Tyler K. L., McPhee D. A., Fields B. N. Distinct pathways of viral spread in the host determined by reovirus S1 gene segment. Science. 1986 Aug 15;233(4765):770–774. doi: 10.1126/science.3016895. [DOI] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Bassel-Duby R., Fields B. N., Tyler K. L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing). J Virol. 1988 Dec;62(12):4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenske E. A., Chanock S. J., Krata L., Fields B. N. Genetic reassortment of mammalian reoviruses in mice. J Virol. 1985 Nov;56(2):613–616. doi: 10.1128/jvi.56.2.613-616.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


