Abstract
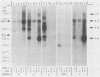
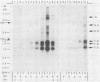
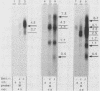
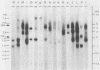
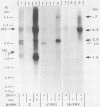
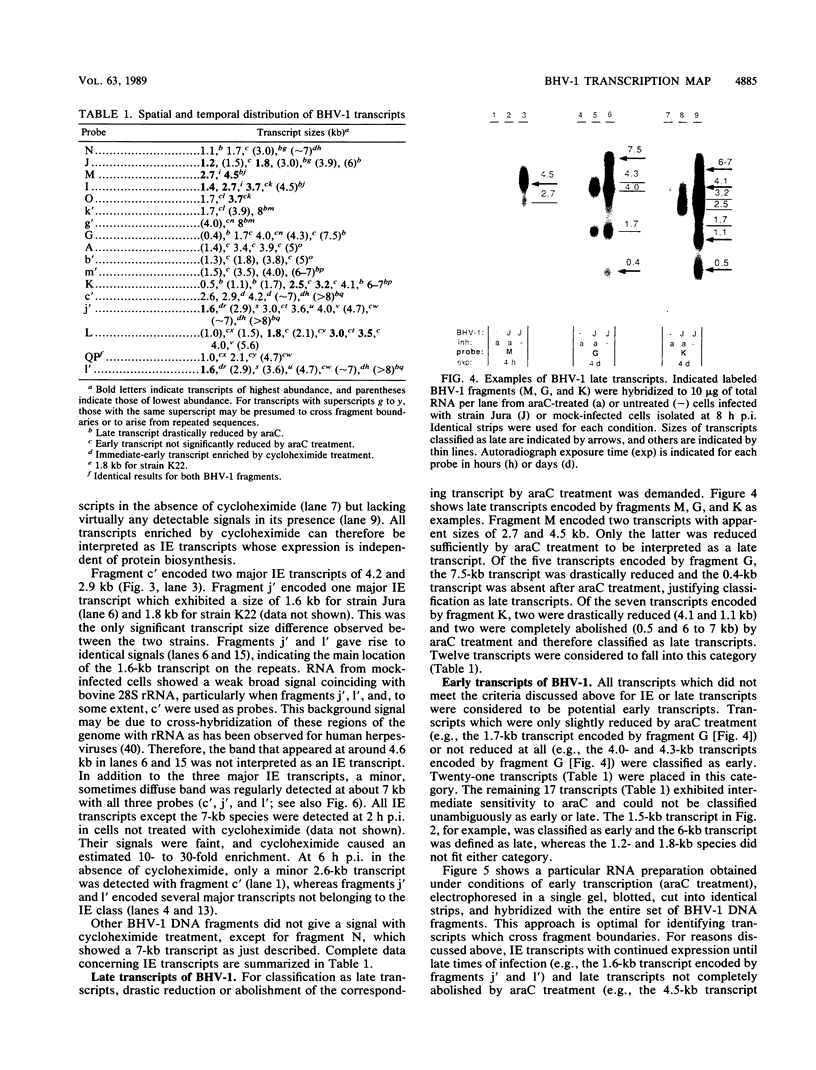
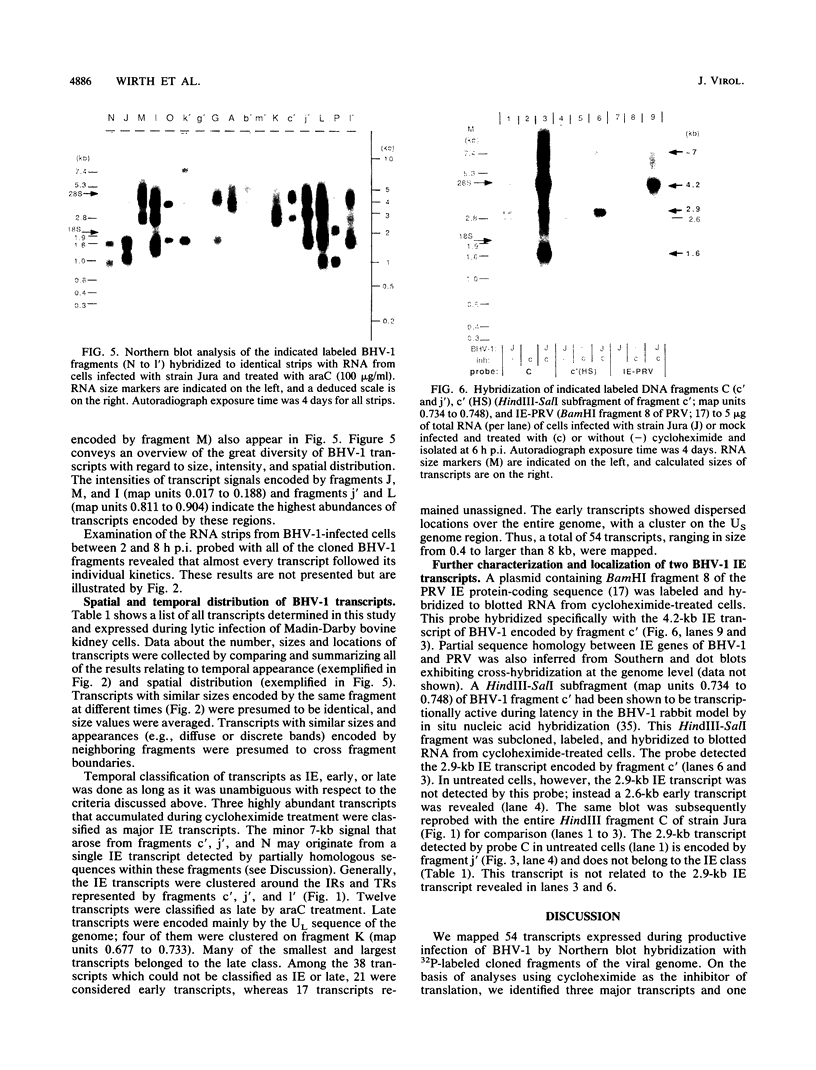
Northern (RNA) blot analysis was used to determine the spatial and temporal distribution of bovine herpesvirus 1 (BHV-1) transcripts. Total RNA was isolated from Madin-Darby bovine kidney cells which had been infected with BHV-1.2b strain K22 or BHV-1.1 strain Jura in the presence or absence of metabolic inhibitors. Cloned restriction fragments representing the entire genome of strain K22 were labeled with 32P and hybridized to immobilized RNA. A total of 54 BHV-1 transcripts were found, ranging in size from 0.4 to larger than 8 kilobases (kb). The inverted repeat regions and an adjacent segment of the unique large part of the BHV-1 genome encoded three major immediate-early (IE) transcripts and one minor IE transcript enriched after cycloheximide treatment of infected cells. Late transcripts were identified by drastically reduced abundance after cytosine arabinoside (araC) treatment. Twelve late transcripts were encoded mainly by the unique long genome region, with a cluster of four transcripts located on HindIII fragment K (map units 0.677 to 0.733). The 21 transcripts unaffected by araC treatment were defined as early; they showed dispersed locations over the whole genome, with a cluster on the unique short sequence. The 17 remaining transcripts could not be classified unambiguously as early or late by these techniques. The IE transcript with a size of 4.2 kb exhibited homology with the single IE gene of pseudorabies virus, and the IE transcript with a size of 2.9 kb was encoded in part by the genome region known to be transcriptionally active during latency.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiuk L. A., Acres S. D., Misra V., Stockdale P. H., De Clercq E. Susceptibility of bovid herpesvirus 1 to antiviral drugs: in vitro versus in vivo efficacy of (E)-5-(2-Bromovinyl)-2'-deoxyuridine. Antimicrob Agents Chemother. 1983 May;23(5):715–720. doi: 10.1128/aac.23.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk L. A., L'Italien J., van Drunen Littel-van den Hurk S., Zamb T., Lawman J. P., Hughes G., Gifford G. A. Protection of cattle from bovine herpesvirus type I (BHV-1) infection by immunization with individual viral glycoproteins. Virology. 1987 Jul;159(1):57–66. doi: 10.1016/0042-6822(87)90347-3. [DOI] [PubMed] [Google Scholar]
- Bello L. J., Whitbeck J. C., Lawrence W. C. Map location of the thymidine kinase gene of bovine herpesvirus 1. J Virol. 1987 Dec;61(12):4023–4025. doi: 10.1128/jvi.61.12.4023-4025.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur T., Moyal M., Rösen-Wolff A., Darai G., Becker Y. Characterization of RNA transcripts from herpes simplex virus-1 DNA fragment BamHI-B. Virology. 1989 Mar;169(1):1–8. doi: 10.1016/0042-6822(89)90034-2. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Veach R. A., Ihara S. Localization of the regions of homology between the genomes of herpes simplex virus, type 1, and pseudorabies virus. Virology. 1983 May;127(1):194–204. doi: 10.1016/0042-6822(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Preston C. M. DNA sequences which regulate the expression of the pseudorabies virus major immediate early gene. Virology. 1987 Apr;157(2):307–316. doi: 10.1016/0042-6822(87)90273-x. [DOI] [PubMed] [Google Scholar]
- Caughman G. B., Robertson A. T., Gray W. L., Sullivan D. C., O'Callaghan D. J. Characterization of equine herpesvirus type 1 immediate early proteins. Virology. 1988 Apr;163(2):563–571. doi: 10.1016/0042-6822(88)90297-8. [DOI] [PubMed] [Google Scholar]
- Engels M., Giuliani C., Wild P., Beck T. M., Loepfe E., Wyler R. The genome of bovine herpesvirus 1 (BHV-1) strains exhibiting a neuropathogenic potential compared to known BHV-1 strains by restriction site mapping and cross-hybridization. Virus Res. 1986 Oct;6(1):57–73. doi: 10.1016/0168-1702(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Everett R. D. The regulation of transcription of viral and cellular genes by herpesvirus immediate-early gene products (review). Anticancer Res. 1987 Jul-Aug;7(4A):589–604. [PubMed] [Google Scholar]
- Fenwick M. L., McMenamin M. Synthesis of alpha (immediate-early) proteins in Vero cells infected with pseudorabies virus. J Gen Virol. 1984 Sep;65(Pt 9):1449–1456. doi: 10.1099/0022-1317-65-9-1449. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., Caughman G. B., O'Callaghan D. J., Staczek J. Regulation of equine herpesvirus type 1 gene expression: characterization of immediate early, early, and late transcription. Virology. 1987 May;158(1):79–87. doi: 10.1016/0042-6822(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Gray W. L., Baumann R. P., Robertson A. T., O'Callaghan D. J., Staczek J. Characterization and mapping of equine herpesvirus type 1 immediate early, early, and late transcripts. Virus Res. 1987 Sep;8(3):233–244. doi: 10.1016/0168-1702(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Ludwig H., Buhk H. J. Specificity of cleavage in replicative-form DNA of bovine herpesvirus 1. J Virol. 1988 Apr;62(4):1355–1363. doi: 10.1128/jvi.62.4.1355-1363.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Unity and diversity in the herpesviruses. J Gen Virol. 1977 Oct;37(1):15–37. doi: 10.1099/0022-1317-37-1-15. [DOI] [PubMed] [Google Scholar]
- Ihara S., Feldman L., Watanabe S., Ben-Porat T. Characterization of the immediate-early functions of pseudorabies virus. Virology. 1983 Dec;131(2):437–454. doi: 10.1016/0042-6822(83)90510-x. [DOI] [PubMed] [Google Scholar]
- KENDRICK J. W., GILLESPIE J. H., MCENTEE K. Infectious pustular vulvovaginitis of cattle. Cornell Vet. 1958 Oct;48(4):458–495. [PubMed] [Google Scholar]
- Lawrence W. C., D'urso R. C., Kundel C. A., Whitbeck J. C., Bello L. J. Map location of the gene for a 130,000-dalton glycoprotein of bovine herpesvirus 1. J Virol. 1986 Nov;60(2):405–414. doi: 10.1128/jvi.60.2.405-414.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S., Courtney R. J., Fowler G., Rouse B. T. Herpes simplex virus type 1-specific cytotoxic T lymphocytes recognize virus nonstructural proteins. J Virol. 1988 Jul;62(7):2265–2273. doi: 10.1128/jvi.62.7.2265-2273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J. E., Good P. J., VanOort H. J., Campbell A. R., Reed D. E. Cloning and cleavage site mapping of DNA from bovine herpesvirus 1 (Cooper strain). J Virol. 1983 Jul;47(1):259–264. doi: 10.1128/jvi.47.1.259-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler A. E., Matile H., Gassmann U., Engels M., Wyler R. European isolates of bovine herpesvirus 1: a comparison of restriction endonuclease sites, polypeptides, and reactivity with monoclonal antibodies. Arch Virol. 1985;85(1-2):57–69. doi: 10.1007/BF01317006. [DOI] [PubMed] [Google Scholar]
- Metzler A. E., Schudel A. A., Engels M. Bovine herpesvirus 1: molecular and antigenic characteristics of variant viruses isolated from calves with neurological disease. Arch Virol. 1986;87(3-4):205–217. doi: 10.1007/BF01315300. [DOI] [PubMed] [Google Scholar]
- Misra V., Blumenthal R. M., Babiuk L. A. Proteins Specified by bovine herpesvirus 1 (infectious bovine rhinotracheitis virus). J Virol. 1981 Nov;40(2):367–378. doi: 10.1128/jvi.40.2.367-378.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Ostrove J. M., Reinhold W., Fan C. M., Zorn S., Hay J., Straus S. E. Transcription mapping of the varicella-zoster virus genome. J Virol. 1985 Nov;56(2):600–606. doi: 10.1128/jvi.56.2.600-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen L. J., Field H. J. Genomic localization and sequence analysis of the putative bovine herpesvirus-1 DNA polymerase gene. Arch Virol. 1988;98(1-2):27–38. doi: 10.1007/BF01321003. [DOI] [PubMed] [Google Scholar]
- Pacha R. F., Condit R. C. Characterization of a temperature-sensitive mutant of vaccinia virus reveals a novel function that prevents virus-induced breakdown of RNA. J Virol. 1985 Nov;56(2):395–403. doi: 10.1128/jvi.56.2.395-403.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold W. C., Straus S. E., Ostrove J. M. Directionality and further mapping of varicella zoster virus transcripts. Virus Res. 1988 Feb;9(2-3):249–261. doi: 10.1016/0168-1702(88)90034-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robertson A. T., Caughman G. B., Gray W. L., Baumann R. P., Staczek J., O'Callaghan D. J. Analysis of the in vitro translation products of the equine herpesvirus type 1 immediate early mRNA. Virology. 1988 Oct;166(2):451–462. doi: 10.1016/0042-6822(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Beam S. L., Mayfield J. E. Mapping bovine herpesvirus type 1 latency-related RNA in trigeminal ganglia of latently infected rabbits. J Virol. 1987 Dec;61(12):3827–3831. doi: 10.1128/jvi.61.12.3827-3831.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Seal B. S., St Jeor S. C., Taylor R. E. Restriction endonuclease analysis of bovine herpesvirus 1 DNA and nucleic acid homology between isolates. J Gen Virol. 1985 Dec;66(Pt 12):2787–2792. doi: 10.1099/0022-1317-66-12-2787. [DOI] [PubMed] [Google Scholar]
- Spector D. J., Jones T. R., Parks C. L., Deckhut A. M., Hyman R. W. Hybridization between a repeated region of herpes simplex virus type 1 DNA containing the sequence [GGC]n and heterodisperse cellular DNA and RNA. Virus Res. 1987 Feb;7(1):69–82. doi: 10.1016/0168-1702(87)90058-x. [DOI] [PubMed] [Google Scholar]
- Vlcek C., Paces V., Schwyzer M. Nucleotide sequence of the pseudorabies virus immediate early gene, encoding a strong transactivator protein. Virus Genes. 1989 Aug;2(4):335–346. doi: 10.1007/BF00684041. [DOI] [PubMed] [Google Scholar]
- Weinheimer S. P., McKnight S. L. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J Mol Biol. 1987 Jun 20;195(4):819–833. doi: 10.1016/0022-2836(87)90487-6. [DOI] [PubMed] [Google Scholar]
- Whitbeck J. C., Bello L. J., Lawrence W. C. Comparison of the bovine herpesvirus 1 gI gene and the herpes simplex virus type 1 gB gene. J Virol. 1988 Sep;62(9):3319–3327. doi: 10.1128/jvi.62.9.3319-3327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Wagner E. K. The kinetics of expression of individual herpes simplex virus type 1 transcripts. Virus Genes. 1987 Nov;1(1):49–60. doi: 10.1007/BF00125685. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Zamb T., Babiuk L. A. Synthesis, cellular location, and immunogenicity of bovine herpesvirus 1 glycoproteins gI and gIII expressed by recombinant vaccinia virus. J Virol. 1989 May;63(5):2159–2168. doi: 10.1128/jvi.63.5.2159-2168.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]