Abstract
We have generated null mutant mice that lack expression of all isoforms encoded by the trkC locus. These mice display a behavioral phenotype characterized by a loss of proprioceptive neurons. Neuronal counts of sensory ganglia in the trkC mutant mice reveal less severe losses than those in NT-3 null mutant mice, strongly suggesting that NT-3, in vivo, may signal through receptors other than trkC. Mice lacking either NT-3 or all trkC receptor isoforms die in the early postnatal period. Histological examination of trkC-deficient mice reveals severe cardiac defects such as atrial and ventricular septal defects, and valvular defects including pulmonic stenosis. Formation of these structures during development is dependent on cardiac neural crest function. The similarities in cardiac defects observed in the trkC and NT-3 null mutant mice indicate that the trkC receptor mediates most NT-3 effects on the cardiac neural crest.
Neurotrophins are members of a highly homologous family of growth factors that contribute to the regulation of proliferation, survival, and differentiation of different cell populations in the mammalian nervous system (1). This family consists of nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), neurotrophin-4/5, and neurotrophin-6 (2, 3). Upon binding to extracellular Ig-like domains of the Trk receptor tyrosine kinases, neurotrophins activate these receptors, thus triggering subsequent biological responses (4, 5). Neurotrophins also interact with the p75 neurotrophin receptor that lacks intrinsic enzymatic activity (6, 7).
In vitro experiments have demonstrated that NGF binds only to trkA (8), and BDNF and neurotrophin-4/5 bind to trkB (9–11). NT-3 binds and signals mainly through trkC (12) but can bind also to trkA and trkB with lower affinity in biochemical assays (5). NT-3 is also able to activate the trkA and trkB receptors in NIH 3T3 cells (13). However, physiological concentrations of NT-3 fail to activate trkA and trkB when these receptors are coexpressed in neural crest-derived cells (PC12 cells) (13). Thus, it has been hypothesized that NT-3-mediated signals in neurons are transmitted specifically by trkC (13).
The pattern of expression of neurotrophins and their receptors in adult and embryonic tissues in rodents, in combination with in vitro and in vivo assays, have helped to predict the cells within sensory and motor ganglia likely to be the targets of neurotrophin action (14–16). In dorsal root ganglia (DRG), correlations have been made between the expression of trk receptors on different soma size neurons and target tissue innervation; coutaneous afferents correlate with small neurons that are trkA positive and muscle afferents correlate with large trkC-expressing neurons (16, 17). However, these studies also suggest that different neurotrophins may act on a single neuronal population during different stages of development. For example, before the axons reach their target fields, neurotrophins may exert local effects (18). Upon reaching their target field, neurons then switch dependence to a different neurotrophin (19, 20). Also, individual neurons can express more than one type of trk receptor, suggesting that neurotrophins could have additive or independent functions on the same cell (17, 21, 22). Furthermore, the wide expression of trkC in many mouse embryonic tissues suggests that NT-3 could have pleiotropic functions during development affecting both neuronal and nonneuronal tissues (23).
Mice lacking individual members of the neurotrophin and trk receptor families have confirmed some of these early hypotheses. In particular, NGF- and trkA-deficient animals have similar neuronal deficits, hypoalgesia, and reductions in the number of small-diameter nociceptive afferents (24, 25). Although BDNF and trkB null mutant animals also exhibit similar deficits, their analysis is complicated by an additional ligand for trkB, neurotrophin-4/5, as well as the persistent expression of truncated isoforms of the trkB receptors (25–29).
The mammalian trkC locus encodes at least eight polypeptides (30, 31). Three of these proteins represent tyrosine kinase receptors that differ in insertions in the kinase domain; the other receptors lack the catalytic kinase domain. It is not clear whether the truncated isoforms can signal or exert effects on trkC-kinase receptors. The survival and neuronal deficits in NT-3 −/− and mice that lack only the kinase active isoforms of trkC (trkC-kinase −/−) are quite different even though both exhibit similar deficiencies in movement and loss of large-diameter muscle afferents projecting to the ventral horn (32–36). The majority of the NT-3-deficient animals die just after birth, whereas the trkC-kinase −/− animals survive up to 3 weeks. Neuronal counts on spinal sensory ganglia show that the loss of neurons is greater in NT-3 −/− than in trkC-kinase −/− mice (32–34). To address these discrepancies between NT-3 and trkC-kinase deficient mice, we have generated and characterized mice with a targeted mutation that disrupts the expression of all trkC receptor proteins including the truncated isoforms (trkC −/−). These trkC −/− mice exhibit the same abnormal posture and limb movements as the trkC-kinase −/− mice previously described (32). However, neuronal counts on neural crest and placode-derived sensory ganglia revealed that the losses of neurons were more severe in the NT-3 −/− mice than in the trkC −/− animals described here. Furthermore, these trkC −/− mice exhibit more severe neuronal losses than those reported for the trkC-kinase −/− animals. Our findings suggest a function for the truncated trkC isoforms in vivo and strongly suggest that NT-3 also activates other “nonpreferred” trk receptors during development.
Most of the trkC −/− mice, like the NT-3 −/− mice, die within the first week of birth. Histological examination of trkC-deficient mice unveiled a nonneuronal function for the trkC gene. trkC −/− mice exhibit a cardiac phenotype similar to the NT-3 −/− animals (37), suggesting that the trkC receptor is the main transducer of NT-3 signaling to support the normal development of the mammalian heart.
MATERIALS AND METHODS
Targeting Vector and Generation of trkC Mutant Mice.
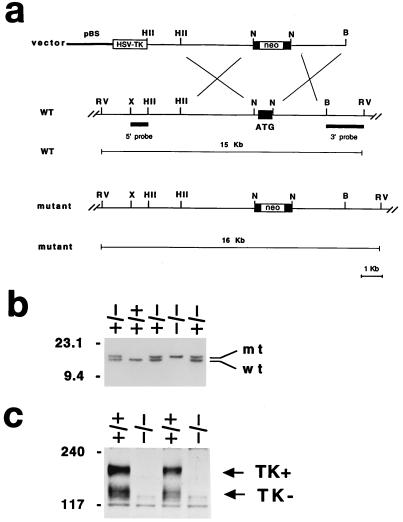
The replacement-type targeting vector consisting of a 9.0-kb 129/SV (Stratagene) mouse genomic fragment, where the exon containing the translation start codon, common to all isoforms, of the trkC locus was replaced with the pGKneobpA cassette used as a positive selectable marker. The pGK-thymidine kinase cassette was introduced as negative selectable marker (Fig. 1a). Electroporation and selection were performed by using the CJ7 embryonic stem (ES) cell line (129/sv) as described (38). Genomic DNA derived from G418/FIAU-resistant ES cell clones were screened by using a diagnostic EcoRV restriction enzyme digestion with the 5′ and 3′ probes external to the targeting vector sequence indicated in Fig. 1b. Recombinant clones containing the expected 16-kb rearrangement were obtained at a frequency of 1/17.
Figure 1.
Generation of trkC-deficient mice. (a) Schematic showing the replacement vector and strategy used to inactivate the trkC locus. Closed bar (ATG) indicates the trkC exon containing the translation start site. Restriction enzyme sites are as indicated. B, BamHI; RV, EcoRV; X, XbaI; HII, HindII; N, NotI. pBS indicates the pBluescript cloning vector (Stratagene). (b) Southern blot analysis of tail DNA from a litter obtained by intercrossing two trkC +/− mice. EcoRV restriction enzyme digestion and the 3′ probe indicated in a were employed to detect rearrangements in the mouse trkC locus. The 15-kb wild-type (wt) and 16-kb rearranged (mt) DNA fragments are indicated; the positions of DNA molecular size markers are indicated on the left. (c) Western blot analysis of trkC protein expression in mutant (−/−) and control (+/+) newborn mouse brains. An antiserum directed against the juxtamembrane domain of the trkC receptor was employed for the analysis. The position of the full-length (TK+) and of the truncated (TK−) trkC proteins are indicated (arrows). Apparent molecular weights are indicated on the left.
Three independent trkC recombinant ES cell clones injected into C57BL/6 blastocysts generated chimeras that transmitted the mutated trkC allele to the progeny when mated to C57BL/6 females. Breeding of two trkC +/− mice gave rise to homozygous mutant mice at a frequency of 25%. Mice were bred in a specific, pathogen-free facility with food and water ad libitum.
Western Blot Analysis.
Glycoproteins were concentrated from Nonidet P-40 lysates of mouse brains with wheat germ agglutinin-Sepharose, resolved on 7% SDS/PAGE, transferred to nitrocellulose, and detected with the antiserum 656 directed against the juxtamembrane domain of the trkC receptor as described (39).
Histologic Techniques.
Heterozygoute (+/−) NT-3 (35) or (+/−) trkC mice were intercrossed by brother/sister matings for histological analysis of pups. The genotype of each newborn mouse was determined by analysis of tail-derived DNA as described (ref. 35; see above). Following decapitation, the bodies and heads of the newborn pups were immediately fixed in Bouin’s fixative overnight. The next day after rinsing for few hours in water the heads and spinal cords were transferred to 70% ethanol and processed for paraffin embedding. The contents of the thoracic cavity were dissected en block prior to embedding in paraffin. For histological examination of the hearts, sections of 5 μm were stained by using hematoxylin/eosin as described (40). For neuronal counts, the heads of one mutant and one wild-type littermate were embedded in the same block, sectioned sagitally at 5 μm, and Nissl-stained with 0.1% cresyl violet. To assure unbiased analysis, neuronal counts were performed in a blinded fashion.
RESULTS
Generation of trkC-Deficient Mice.
To disrupt all the isoforms encoded by the trkC locus, we constructed a vector in which part of the exon containing the translation start (ATG) codon was removed and replaced with the neo gene used as selectable marker (Fig. 1a). The resultant targeted allele was identified by Southern blot analysis (Fig. 1b). Western blot analysis of protein extracts from trkC mutant mouse brains confirmed the complete disruption of the trkC locus because both full-length and truncated isoforms could not be detected by an antibody specific for the common juxtamembrane domain of the trkC proteins (Fig. 1c). Furthermore, we did not detect any trkC mRNA by reverse transcription–PCR analysis (data not shown).
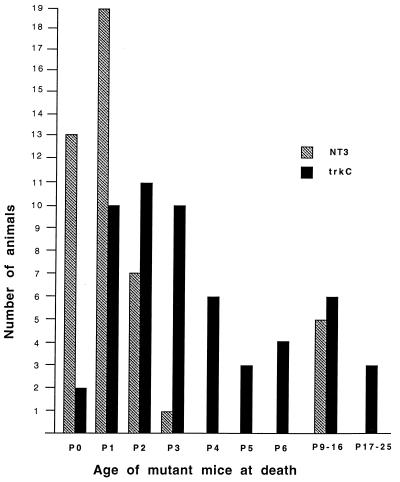
Mutant mice obtained by mating mice heterozygous for either the trkC or the NT-3 (35) targeted mutation were scored for their survival rate (Fig. 2). Both trkC- and NT-3-deficient mice were generated in the same genetic strain background (129/C57bl/6) and maintained in the same facility under identical conditions. No significant differences in litter size were detected between the two mouse lines. However, there were significant differences in life span of 55 trkC −/− and 44 NT-3 −/− mice. As shown in Fig. 2, 30% of NT-3 −/− mice die immediately after birth whereas less than 5% of trkC mutant mice succumb in the first few hours postnatally. Furthermore, almost 90% of the NT-3 knockout mice die by postnatal day 2 (P2), whereas equivalent lethality in trkC mutant mice is not reached until postnatal day 6 (P6) (Fig. 2). To further ensure uniformity of conditions, mutant mice were also obtained by mating mice heterozygous in both the trkC +/− and NT-3 +/− locus. Similar to the data in Fig. 2, NT-3 mutant mice showed reduced survival compared with their trkC −/− littermates (data not shown). Thus, mice lacking all trkC isoforms live slightly longer than the NT-3 −/− ones. However, trkC −/− display a more severe phenotype than the trkC-kinase −/− animals that retain expression of the truncated isoforms and that live up to 4 weeks (32). Considering that both mutations were derived in a similar genetic background (129/C57bl/6), these results suggest that truncated trkC receptors may play a role in mouse development.
Figure 2.
Survival rate of NT-3 and trkC mutant mice. Bar chart indicating the number of NT-3 and trkC null mice that died at the specific age indicated (P, postnatal day). No mutant animal lived longer than 25 days.
NT-3 −/− Mice Exhibit a More Severe Loss of Sensory Neurons than trkC −/− Mice.
Mice lacking all trkC receptors display the same behavioral phenotype attributed to defects in proprioception observed in mice lacking either the full-length trkC tyrosine kinase receptors or NT-3 (32–36). Retrograde labeling of sensory and motor neurons by using the lipid-soluble fluorescent tracer DiI in axial muscle at the lumbar level of trkC mutant mice confirmed the loss of Ia proprioceptive neuronal fibers that terminate in lamina IX of the spinal cord where they contact motor neurons to form the reflex arc (ref. 35; data not shown). Previous reports showed a reduced neuronal loss in the DRG of the trkC-kinase −/− mice relative to the ligand NT-3-deficient mice (32, 41). However, neuronal cell counts of other sensory ganglia from trkC-kinase −/− mice were not reported.
To rule out differences in neuronal cell counts caused by genetic background and/or methodology of analysis, we evaluated in parallel the trkC −/− and NT-3 −/− mutant mice for loss of peripheral sensory and sympathetic neurons (Table 1). Neuronal cell counts of six different sensory ganglia were performed on individual newborn animals to allow direct comparisons also with the other neurotrophin and trk receptor knockouts that have been published recently (28, 29, 34, 42). As shown in Table 1, we confirmed the severe neuronal losses in the different sensory ganglia of NT-3 −/− mice including the DRG at L5 level, trigeminal, cochlear, vestibular, geniculate, and petrosal-nodose. For example, we observed a 69% loss in L5 DRGs consistent with previous reports (33, 34). In the same ganglia the trkC −/− mice revealed less severe neuronal losses (33% in L5); yet, this reduction is significantly higher than that reported previously for the trkC-kinase −/− animals (17%) at P1 (41). Similarly, we observed a more severe neuronal loss in the cochlear ganglia of the trkC −/− mice (≈70%) relative to the trkC-kinase −/− animals (51%; ref. 41). Yet, we confirmed the severe loss (87%) reported for the NT-3 −/− mice (85%; refs. 33 and 34). However, some ganglia demonstrated similar losses in the trkC −/− and the trkC-kinase −/− mice. In the trigeminal ganglia, reduction by 20% is noted in both lines and in the vestibular ganglia similar neuronal losses of ≈15% are found (refs. 41 and 43; Table 1). Taken together, these data suggest that the truncated trkC isoform may play a role, at least in certain sensory ganglia. However, unless a direct comparison between the two mutant animals is performed, it is difficult to discern if the differences observed are significant and not caused by the criteria used for counting neurons or are caused by the presence of nontyrosine kinase forms of trkC.
Table 1.
Neuronal cell counts in sensory and sympathetic populations of wild type, trkC, and NT-3 mutant mice
| Wild type (5) | trkC −/− (5) | Reduction, % | |
| Sensory ganglia | |||
| Trigeminal | 50,646 ± 353 | 39,912 ± 638 | 21*** |
| Geniculate | 1,569 ± 132 | 1,419 ± 85 | 11 |
| Vestibular | 4,324 ± 106 | 3,668 ± 60 | 15*** |
| Cochlear | 8,761 ± 644 | 2,611 ± 162 | 70*** |
| Petrosal-nodose | 10,084 ± 353 | 8,659 ± 296 | 14*** |
| L5 dorsal root (3) | 7,157 ± 587 | 4,770 ± 337 | 33** |
| Sympathetic ganglion | |||
| Superior cervical (3) | 25,084 ± 2,140 | 25,956 ± 1,912 | 0 |
| Wild type (3) | NT 3 −/− (3) | Reduction, % | |
| Sensory ganglia | |||
| Trigeminal | 39,765 ± 1,334 | 14,808 ± 953 | 62*** |
| Geniculate | 1,465 ± 135 | 949 ± 85 | 35* |
| Vestibular | 4,110 ± 335 | 3,352 ± 325 | 19 |
| Cochlear | 7,905 ± 331 | 980 ± 270 | 87*** |
| Petrosal-nodose | 9,724 ± 1,118 | 6,396 ± 724 | 34** |
| L5 dorsal root | 7,107 ± 627 | 2,222 ± 173 | 69*** |
| Sympathetic ganglion | |||
| Superior cervical | 23,862 ± 1,550 | 13,578 ± 1,896 | 44*** |
Tissue sections of newborn (P0) wild type and mutant littermates were prepared as described. Neurons having a clear nucleus and nucleolus were counted in every sixth section, and the sum of counts was multiplied by 6. Values are not corrected and are expressed as mean number of neurons ± SEM. Differences were evaluated using a one-tailed Student’s test; *P < 0.01, **P < 0.02, ***P < 0.005. In parenthesis, the numbers of animals analyzed are indicated. Wild-type mice derived from intercrosses of heterozygous trkC or NT-3 mice showed a significant difference in neuronal counts of the trigeminal ganglion. We hypothesize that this is caused by a maternal effect of reduced circulating NT-3 in the pregnant NT-3 +/− mice.
Information on neuronal cell counts in the geniculate and petrosal-nodose ganglia have not been published for the trkC-kinase −/− mice. However, comparisons can be made with the NT-3 −/− mice (Table 1). Significant differences in reduction of neurons in both geniculate and petrosal-nodose neuronal losses were noted between the NT-3 −/− mice (35% and 34%) and the trkC −/− mice (11% and 14%).
We also evaluated the superior cervical ganglion, because NT-3 is known to play an important role in its development and can support sympathetic neuroblasts in vitro (33, 34, 44, 45). At early stages of development, only trkC is expressed in this ganglion (46). At later stages, postmitotic neurons express trkA and require NGF as a target derived neurotrophin (46, 47). Considering the mediation of NT-3 activity by trkC in early sympathetic neuroblasts, we expected to see similar neuronal losses in NT-3 −/− and trkC −/− mice (≈50%). However, as reported for trkC-kinase −/− mice (46), we did not see any reduction at all in trkC −/− mice (Table 1). These data support the notion that, in vivo, NT-3 might act at later stages of sympathetic ganglion development through trkA. Furthermore, a recent report showed that trkC-kinase −/− sympathetic neurons in culture can be rescued by adding NT-3 to the culture media (48). Although saturating amounts of the neurotrophin were used in this experiment, this data also suggested that NT-3 can act through other receptors during neuronal development.
Cardiac Defects in trkC-Deficient Mice.
In rodents, neurotrophins and their receptors are expressed in many developing organs. In particular, the presence of both NT-3 and trkC mRNAs has been reported in the heart, the aorta and putative migrating neural crest cells (23, 31, 40, 49, 50). Recently, we have shown that mice lacking NT-3 have severe defects in cardiac structures of neural crest origin including atrial and ventricular dilatation, atrial and ventricular septal defects, and abnormalities of valvular architecture (37). To establish whether the defects found in the NT-3 −/− mouse are caused by a failure in activating the trkC receptor, we analyzed the hearts of eight trkC-deficient mice.
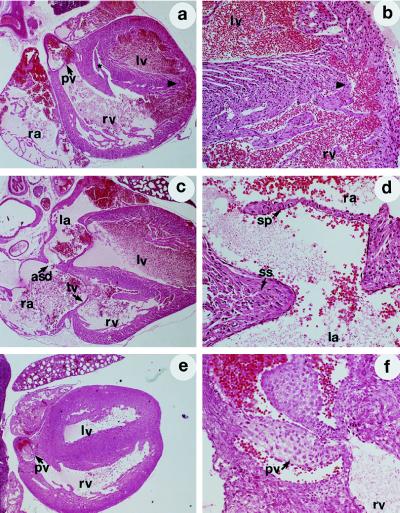
Examination of the thoracic cavity of these animals revealed markedly enlarged and globular hearts with congested, hemorrhagic lungs. Microscopic evaluation displayed valvular defects in all animals, with hypertrophy/hyperplasia of the pulmonic valve occurring most frequently (3/8 of the trkC −/− animals; Table 2; Fig. 3 e and f). In addition, there was hypertrophy and thickening of the pulmonary veins as they branch within the lung parenchyma. Defects in chamber septation were detected in the majority of the animals with 4/8 demonstrating large atrial secundum defects, and 3/8 with large ventricular septal defects (Fig. 3 a–d). Abnormalities in the development or septation of the cardiac outflow tracts were also noted, with the identification of one animal with an apparent truncus arteriosus (incomplete septation of the aorta and pulmonary artery). All animals exhibited significant right ventricular chamber enlargement, although this may represent a secondary defect given the severity of associated valvular and septa abnormalities (Table 2).
Table 2.
Heart abnormalities in trkC-deficient animals
| Defects | trkC −/− | Wild type |
|---|---|---|
| Globular heart | 8/8 | 0/6 |
| ASD | 4/4 | 0/6 |
| VSD | 3/8 | 0/6 |
| Pulmonic stenosis | 3/8 | 0/6 |
| Truncus arteriosis | 1/8 | 0/6 |
| Valvular defects (AV, MV, TV, PV) | 8/8 | 0/6 |
ASD, atrial septal defect; VSD, ventricular septal defects; AV, aortic valve; MV, mitral valve; TV, tricuspid valve; PV, pulmonic valve.
Figure 3.
Malformations in cardiogenesis of trkC −/− mice. Representative hematoxylin/eosin stained sections of newborn trkC −/− mice (P0). The genotype of each animal was determined by Southern blot analysis. (a) Section of a heart demonstrating ventricular septa defects (∗ and arrowhead). (×25.) (b) Higher magnification (×75) of a, with ventricular septa defect (arrowhead) indicated. (c) Atrial septa defect (asd) in a trkC −/− mouse heart. (d) Higher magnification (×75) of c with septum primum (sp) and septum secundum (ss) indicated. (e) Section demonstrating thickening of pulmonic valve leaflets (pv). (×25.). (f) Higher magnification of e (×75), with thickening of pulmonic valve leaflet indicated. rv, Right ventricle; lv, left ventricles; pv, pulmonary valve; tv, tricuspid valve; ra, right atrium; la, left atrium.
The frequency, severity, and type of cardiovascular defects in the trkC −/− animals are similar to those described in the NT-3 −/− animals (37). These results suggest that trkC mediates most NT-3 signaling in cardiac development. Furthermore, the presence of trkC receptors in neural crest cells migrating during embryogenesis and the identification of cardiac defects in structures of neural crest origin is consistent with a direct role for trkC in the cardiac neural crest migration (23, 37).
DISCUSSION
In vitro experiments have demonstrated that NT-3 activates most effectively the trkC receptor tyrosine kinase, but it can also signal through trkA and trkB under certain experimental conditions (13). Whether these interactions occur also in vivo and have physiological relevance is unresolved (51). The availability of trk and neurotrophin null mutant mice has provided a tool to investigate the significance of these ligand/receptor interactions in vivo. NT-3-deficient mice have been independently generated by several laboratories. Mice lacking the kinase active isoforms of trkC have been reported by Klein and colleagues (32) who observed a less severe phenotype in these mice compared with NT-3 null animals (41, 51). To date, it has been unclear whether this discrepancy was caused by the presence of the noncatalytic isoforms of the trkC receptor. Thus, we have generated mice that lack expression of all the kinase active and truncated isoforms of trkC. The present study is a direct comparison of neuronal deficits in mice lacking either the ligand NT-3 or the receptor trkC. We have confirmed previously published reports on neuronal cell losses in both sensory and sympathetic neuronal populations of NT-3 null mice (33, 34). Furthermore, we obtained similar neuronal cell counts in trigeminal, vestibular, and superior cervical ganglia as have been reported for mice lacking only kinase active trkC isoforms (41, 46, 48). However several significant differences were observed: trkC −/− mice live significantly shorter than trkC-kinase −/− mice, although both lines were generated in the same genetic background (32); neuronal cell losses in ganglia such as vestibular and DRGs were more severe in trkC −/− deficient mice. These differences suggest that, in mouse, truncated trkC receptors play a role in the development of subpopulations of sensory neurons and possibly other structures.
At least one isoform of the truncated trkC receptors has an intracellular domain that is highly conserved between mammals and birds, suggesting a signaling function, as a docking site for proteins within a signal transduction pathway involved in neurogenesis (30, 31, 52). However, the truncated isoform(s) could also have roles in sequestering ligand or in regulating full length receptor functions by forming heterodimers of truncated and kinase active trkC isoforms in the presence of the ligand. These later hypotheses are supported by our recent finding that mice which overexpress a truncated isoform of the trkC receptor develop a phenotype similar to the NT-3 −/− mice. These results suggest that the receptor could sequester NT-3 or act in a dominant negative manner to suppress signaling by the kinase active trkC isoforms (M.E.P. and L.T., unpublished data). Other studies investigating the role of truncated trkB receptors support such ideas. In particular, the expression pattern of a truncated trkB receptor in the chicken developing inner ear suggests that truncated trkB molecules form an efficient and selective barrier preventing the diffusion of BDNF and eliminating it by internalization (53). Furthermore, truncated trkB receptors expressed in Xenopus oocytes, function as dominant negative in a heterodimer with the full-length isoform inhibiting the BDNF signaling (54).
Regardless of the differences observed between mice lacking all trkC receptors or only the kinase active isoforms, both lines display significantly less severe neuronal cell losses in almost all ganglia compared with the defects in NT-3 −/− animals. Thus, our data strongly support the notion that NT-3 acts also through other trk receptors in neuronal development.
In particular, we found a 70% reduction of DRG neurons at P0 in NT-3-deficient mice, whereas trkC −/− mice are missing only 33% of these neurons. Other groups have studied extensively the embryonic DRG of NT-3 −/− mice and proposed that in these embryos the precursors cells at embryonic day (E) 11 to E12 undergo apoptosis, accounting for the 70% neuronal cell losses (55). Because only a fraction of DRG neurons are missing in trkC mutant mice, it has been suggested that NT-3 could support DRG neurons by activating trkA or trkB, although this effect could be artificial because of the absence of trkC (51). White et al. (51) proposed that as the onset of survival-dependence on NGF and trkA signaling is concurrent and of equal magnitude at E13.5, NT-3 alone could not support DRG neurons via trkA in the wild-type embryo. However, a recent study by Fariñas et al. (57) provides a more complicated scenario for survival-dependence of early DRG neurons. At E11, they observe an increase in apoptosis of DRG neurons in NT-3 null mutant mice, and suggest that these could be proprioceptive neurons, because they are formed first in the DRG (56). At E12 in the DRGs of NT-3 −/− mice, the precursor cells display proliferation and survival rates that match those of wild-type animals. Furthermore, there is a dramatic increase in the accumulation of neurons in the ganglia of NT-3 mutant animals, suggesting that lack of NT-3 causes precursors cells to prematurely exit the cell cycle. Prematurely differentiated neurons would then undergo massive cell death at E12–13 (51, 57). Thus, contrary to the report by ElShamy and Ernfors (55), Fariñas et al. (57) suggest that neurons and not precursor cells undergo apoptosis. These authors also suggest that there are several temporal waves of neurogenesis in the DRG. The first population of cells affected in the NT-3 −/− mice at E11 could be trkC-expressing neurons (proprioceptive). The second population affected in the NT-3 −/− animals at E12–E13 might express a trk receptor other than trkC. NT-3 can activate trkA in certain cellular backgrounds and is produced by the mesenchyme surrounding the DRGs by E11–E12 (13, 57). In addition, local concentrations of NT-3 could reach levels sufficient to activate trkA expressed in neuronal cells. Lastly, a population of DRG neuron at E11.5 express the trkA receptor and could represent the second population affected in the NT-3 −/− mice (35, 51). In view of these considerations and our current results, we suggest that NT-3 activates the trkA receptor in vivo. However, such an effect may be restricted to a specific developmental stage of the DRG.
We and others have reported expression of neurotrophins, and their cognate trk receptors, in the vascular system (23, 40, 49, 50, 58). Recently, we have shown that NT-3-deficient mice exhibit severe cardiac abnormalities including atria and ventricular septa defects and abnormal valvular architecture. Furthermore, we have observed decreased levels of trkC expression in the hearts of NT-3 −/− embryos, suggesting that the absence of NT-3 expression could result in a reduction of trkC expressing populations within the developing cardiovascular system (37). Our current findings show that the trkC −/− animals have cardiac abnormalities similar to the ones identified in the NT-3 −/− mice. Thus, these defects establish trkC as the most likely transducer of NT-3 action in heart development. The cardiac defects in both null mutant mice involve structures of neural crest origin indicating that NT-3 and trkC are essential for regulating the development of cardiac neural crest. Further studies of embryonic stages will be required to determine whether NT-3/trkC signaling regulates the migration, proliferation, and/or survival of the neural crest cells. Furthermore, the results suggest that in cardiac crest development, NT-3 does not function effectively throw less preferred trk receptors, trkA or trkB.
The trkC-deficient mice have a less severe neuronal phenotype than NT-3-deficient mice, and to date we have not established whether there are differences in the cardiac innervation between the two lines. More specific analysis will address this issue and may identify this mouse as an animal model complementary to the NT-3 −/− for the study of human cardiac congenital abnormalities.
Acknowledgments
We thank Marie Mazzulla, Susan Reid, James Resau, and Eric Klineberg for excellent technical assistance; Isabel Fariñas and Luis Reichardt for sharing their unpublished results; and Esta Sterneck for critical reading of the manuscript. This work was supported by the National Cancer Institute, under contract with ABL (L.T., P.T., L.F.P., J.B.F., and M.E.P.), and by the New York Heart Association and the Hirschl-Weill/Caullier Trust (B.L.H.). B.L.H. is an Established Investigator of the American Heart Association.
ABBREVIATIONS
- NGF
nerve growth factor
- BDNF
brain-derived neurotrophic factor
- NT-3
neurotrophin-3
- DRG
dorsal root ganglia
References
- 1.Lewin G R, Barde Y-A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 2.Barde Y-A. Prog Growth Factor Res. 1990;2:237–248. doi: 10.1016/0955-2235(90)90021-b. [DOI] [PubMed] [Google Scholar]
- 3.Gotz R, Koster R, Winkler C, Raulf F, Lottspeich F, Schartl M, Thoenen H. Nature (London) 1994;372:266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- 4.Perez P, Coll P M, Hempstead B L, Martin-Zanca D, Chao M V. Mol Cell Neurosci. 1995;6:97–105. doi: 10.1006/mcne.1995.1010. [DOI] [PubMed] [Google Scholar]
- 5.Urfer R, Tsoulfas P, O’Connell L, Shelton D L, Parada L F, Presta L G. EMBO J. 1995;14:2795–2805. doi: 10.1002/j.1460-2075.1995.tb07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greene L A, Kaplan D R. Curr Opin Neurobiol. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 7.Chao M V, Hempstead B L. Trends Neurosci. 1995;18:321–326. [PubMed] [Google Scholar]
- 8.Kaplan D R, Hempstead B L, Martin-Zanca D, Chao M V, Parada L F. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 9.Soppet D, Escandon E, Maragos J, Middlemas D S, Reid S W, Blair J, Burton L E, Stanton B R, Kaplan D R, Hunter T, Nikolics K, Parada L F. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Nanduri V, Jing S, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones K R, Reichardt L F, Barbacid M. Cell. 1991;66:395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Lamballe F, Bryant S, Barbacid M. Neuron. 1992;8:947–956. doi: 10.1016/0896-6273(92)90209-v. [DOI] [PubMed] [Google Scholar]
- 12.Lamballe F, Klein R, Barbacid M. Cell. 1991;66:967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- 13.Ip N Y, Stitt T N, Tapley P, Klein R, Greene L A, Barbacid M, Yancopoulos G D. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- 14.Merlio J-P, Ernfors P, Jaber M, Persson H. Neuroscience. 1992;51:513–532. doi: 10.1016/0306-4522(92)90292-a. [DOI] [PubMed] [Google Scholar]
- 15.Schecterson L C, Bothwell M. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- 16.Mu X, Silos-Santiago I, Carrol S, Snider W D. J Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon S B, Armanini M P, Ling L H, Phillips H S. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 18.Davies A M. J Neurobiol. 1994;25:1334–1348. doi: 10.1002/neu.480251103. [DOI] [PubMed] [Google Scholar]
- 19.Buchman V L, Davies A M. Development (Cambridge, UK) 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- 20.Paul G, Davies A M. Dev Biol. 1995;171:590–605. doi: 10.1006/dbio.1995.1307. [DOI] [PubMed] [Google Scholar]
- 21.Kokaia Z, Bengzon J, Metsis M, Kokaia M, Persson H, Lindvall O. Proc Natl Acad Sci USA. 1993;90:6711–6715. doi: 10.1073/pnas.90.14.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miranda R C, Sohrabji F, Toran-Allerand C D. Proc Natl Acad Sci USA. 1993;90:6439–6443. doi: 10.1073/pnas.90.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tessarollo L, Tsoulfas P, Martin-Zanca D, Gilbert D J, Jenkins N A, Copeland N, Parada L F. Development (Cambridge, UK) 1993;118:463–475. doi: 10.1242/dev.118.2.463. [DOI] [PubMed] [Google Scholar]
- 24.Smeyne R J, Klein R, Schnapp A, Long L K, Bryant S, Lewin A, Lira S A, Barbacid M. Nature (London) 1994;368:246–248. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 25.Jones K R, Fariñas I, Backus C, Reichardt L F. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein R, Smeyne R J, Wurst W, Long L K, Auerbach B A, Joyner A L, Barbacid M. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- 27.Ernfors P, Lee K-F, Jaenisch R. Nature (London) 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Ernfors P, Wu H, Jaenisch R. Nature (London) 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- 29.Conover J C, Erickson J T, Katz D M, Bianchi L M, Poueymirou W T, McClain J, Pan L, Helgren M, Ip N Y, Boland P, Friedman B, Wiegand S, Vejsada R, Kato A C, DeChiara T M, Yancopoulos G D. Nature (London) 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela D M, Maisonpierre P C, Glass D J, Rojas E, Nuñez L, Kong Y, Gies D R, Stitt T N, Ip N Y, Yancopoulos G D. Neuron. 1993;10:963–974. doi: 10.1016/0896-6273(93)90211-9. [DOI] [PubMed] [Google Scholar]
- 31.Tsoulfas P, Soppet D, Escandon E, Tessarollo L, Mendoza-Ramirez J-L, Rosenthal A, Nikolics K, Parada L F. Neuron. 1993;10:975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- 32.Klein R, Silos-Santiago I, Smeyne R J, Lira S A, Brambilla R, Bryant S, Zhang L, Snider W D, Barbacid M. Nature (London) 1994;368:249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- 33.Ernfors P, Lee K-F, Kucera J, Jaenisch R. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 34.Fariñas I, Jones K R, Backus C, Wang X-Y, Reichardt L F. Nature (London) 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 35.Tessarollo L, Vogel K S, Palko M E, Reid S W, Parada L F. Proc Natl Acad Sci USA. 1994;91:11844–11848. doi: 10.1073/pnas.91.25.11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tojo H, Kaisho Y, Nakata M, Matsuoka K, Kitagawa M, Abe T, Takami K, Yamamoto M, Shino A, Igarashi K, Aizawa S, Shiho O. Brain Res. 1995;669:163–175. doi: 10.1016/0006-8993(94)01219-8. [DOI] [PubMed] [Google Scholar]
- 37.Donovan M J, Hahn R, Tessarollo L, Hempstead B L. Nat Genet. 1996;14:210–213. doi: 10.1038/ng1096-210. [DOI] [PubMed] [Google Scholar]
- 38.Swiatek P J, Gridley T. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 39.Tsoulfas P, Stephens R M, Kaplan D R, Parada L R. J Biol Chem. 1996;271:5691–5697. doi: 10.1074/jbc.271.10.5691. [DOI] [PubMed] [Google Scholar]
- 40.Donovan M J, Miranda R C, Kraemer R, McCaffrey T A, Tessarollo L, Mahadeo D, Sharif S, Kaplan D R, Tsoulfas P, Parada L F, Toran-Allerand C D, Hajjar D P, Hempstead B L. Am J Pathol. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- 41.Minichiello L, Piehl F, Vasquez E, Schimmang T, Hökfelt T, Represa J, Klein R. Development (Cambridge, UK) 1995;121:4067–4075. doi: 10.1242/dev.121.12.4067. [DOI] [PubMed] [Google Scholar]
- 42.Snider W D. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 43.Pinon L G, Minichiello L, Klein R, Davies A M. Development (Cambridge, UK) 1996;122:3255–3261. doi: 10.1242/dev.122.10.3255. [DOI] [PubMed] [Google Scholar]
- 44.Birren S J, Lo L, Anderson D J. Development (Cambridge, UK) 1993;119:597–610. doi: 10.1242/dev.119.3.597. [DOI] [PubMed] [Google Scholar]
- 45.DiCicco-Bloom E, Friedman W J, Black I B. Neuron. 1993;11:1101–1111. doi: 10.1016/0896-6273(93)90223-e. [DOI] [PubMed] [Google Scholar]
- 46.Fagan A M, Zhang H, Landis S, Smeyne R J, Silos-Santiago I, Barbacid M. J Neurosci. 1996;16:6208–6218. doi: 10.1523/JNEUROSCI.16-19-06208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levi-Montalcini R, Booker B. Proc Natl Acad Sci USA. 1960;46:384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies A M, Minichiello L, Klein R. EMBO J. 1995;14:4482–4489. doi: 10.1002/j.1460-2075.1995.tb00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scarisbrick I A, Jones E G, Isackson P J. J Neurosci. 1993;13:875–893. doi: 10.1523/JNEUROSCI.13-03-00875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hiltunen J O, Arumae U, Moshnyakov M, Saarma M. Circ Res. 1996;79:930–939. doi: 10.1161/01.res.79.5.930. [DOI] [PubMed] [Google Scholar]
- 51.White F A, Silos-Santiago I, Molliver D C, Nishimura M, Philips H, Barbacid M, Snider W D. J Neurosci. 1996;16:4662–4672. doi: 10.1523/JNEUROSCI.16-15-04662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garner A S, Large T H. Neuron. 1994;13:457–472. doi: 10.1016/0896-6273(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 53.Biffo S, Offenhauser N, Carter B D, Barde Y A. Development (Cambridge, UK) 1995;121:2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- 54.Eide F F, Vining E R, Eide B L, Zang K, Wang X-Y, Reichardt L F. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.ElShamy W M, Ernfors P. Neuron. 1996;16:963–972. doi: 10.1016/s0896-6273(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 56.Lawson S N, Biscoe T J. J Neurocytol. 1979;8:265–274. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- 57.Fariñas I, Yoshida C K, Backus C, Reichardt L F. Neuron. 1996;17:1065–1078. doi: 10.1016/s0896-6273(00)80240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamballe F, Smeyne R J, Barbacid M. J Neurosci. 1994;14:14–28. doi: 10.1523/JNEUROSCI.14-01-00014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]