Abstract
Our current understanding of the sound-generating mechanism in the songbird vocal organ, the syrinx, is based on indirect evidence and theoretical treatments. The classical avian model of sound production postulates that the medial tympaniform membranes (MTM) are the principal sound generators. We tested the role of the MTM in sound generation and studied the songbird syrinx more directly by filming it endoscopically. After we surgically incapacitated the MTM as a vibratory source, zebra finches and cardinals were not only able to vocalize, but sang nearly normal song. This result shows clearly that the MTM are not the principal sound source. The endoscopic images of the intact songbird syrinx during spontaneous and brain stimulation-induced vocalizations illustrate the dynamics of syringeal reconfiguration before phonation and suggest a different model for sound production. Phonation is initiated by rostrad movement and stretching of the syrinx. At the same time, the syrinx is closed through movement of two soft tissue masses, the medial and lateral labia, into the bronchial lumen. Sound production always is accompanied by vibratory motions of both labia, indicating that these vibrations may be the sound source. However, because of the low temporal resolution of the imaging system, the frequency and phase of labial vibrations could not be assessed in relation to that of the generated sound. Nevertheless, in contrast to the previous model, these observations show that both labia contribute to aperture control and strongly suggest that they play an important role as principal sound generators.
The study of vocal communication in songbirds makes significant contributions to various biological disciplines (1, 2) and provides inspiration to related areas, such as linguistics (3, 4). However, unlike the case in human speech (5), the physical mechanism of phonation in birds is poorly understood (6–7).
Sound production in songbirds is commonly believed to involve a constriction of the bronchial lumen by the lateral labium (LL), which, when combined with high subsyringeal air sac pressure and increased air velocity, induces vibrations of the medial tympaniform membranes (MTM) by Bernoulli forces and pressure differences. Support for this interpretation was provided by direct observation of vibrations of the MTM in the excised syrinx during artificially induced sound production (8), indirect anatomical and physiological observations (8–17), acoustic analyses (10, 18), simple syringeal models (18), and theoretical accounts (19–23). Detailed analyses of the vocal mechanism in non-songbirds (24–29) also provided a framework for the development of this classical model of songbird phonation. However, unlike the case in non-songbirds (25–27), the predictions of this model for songbirds never have been tested by manipulation of the presumed sound generators, the MTM.
Here we report the results of experiments in which the MTM were surgically disabled. Furthermore, we studied the intact songbird syrinx in situ by filming it through a fine endoscope during sound generation. A similar approach with the less complex syrinx of a non-songbird, the pigeon (Columba livia), revealed a different model of phonation for that species (30). However, in pigeons, vocalizations were artificially generated by injecting gas into the air sac system. To study natural vocalizations in songbirds, the endoscopic technique had to be applied in birds that either spontaneously generated sounds or were induced to vocalize by electrical brain stimulation. The results of this research clearly show that the classical model of songbird phonation has to be revised.
MATERIALS AND METHODS
Animals and Surgery.
A hand-raised female American crow (Corvus brachyrhynchus) was used to study spontaneous vocalizations, and wild-caught male birds (three Northern cardinals, Cardinalis cardinalis, and four brown thrashers, Toxostoma rufum) were used to study brain-stimulated vocalizations. The crow was anesthetized with isoflurane to expose the trachea 2–3 cm below the glottis. A small opening was cut into the ventral side of the trachea by partially removing a tracheal cartilage. During recording, the crow was awake, wrapped in cloth, and fitted with a combined collar and beak holder made of Plexiglas to avoid damage to the fiber scope. For acute brain stimulation experiments, the birds were anesthetized with chloropent (i.m. injections of 3.9 μl/g and 3.6 μl/g for thrashers and cardinals, respectively), and the trachea was opened as in the crow. In addition, one of the thoracic air sacs was cannulated by inserting a flexible tube (Dow Corning, type 508-005), which was connected to a piezoresistive pressure transducer (Fujikura, type FPM-02PG).
The MTM were fully disabled in four male zebra finches (Taeniopygia guttata) and three male cardinals. After recording of control song, birds were anesthetized with isoflurane, and the syrinx was exposed by opening the interclavicular air sac. The MTM were penetrated with the fine tips of a pair of forceps, which then were opened repeatedly with different orientation, tearing them across their entire width. The air sac membrane then was closed with surgical suture and tissue adhesive. All birds resumed singing within 2 days, and song was recorded between 1 and 5 days after the surgery. Because it was impossible to visualize the cranial and dorsal portions of the MTM during the surgery, birds were sacrificed at the end of the experiment and dissected by an independent observer to determine the extent of damage to the MTM. Experiments were included in this study only if the MTM on both sides were fully ruptured and withdrawn to the perimeter of cartilaginous attachment, i.e., the entire area previously covered by the membrane was replaced by a hole.
Call Generation.
Spontaneous crow calls were induced by verbal stimulation or tongue clicks. In cardinals and brown thrashers phonation was induced by placing the bird in a stereotaxic apparatus, making a small opening in the skull, and stimulating the left high vocal center through bipolar tungsten electrodes (Grass stimulator, type S88, and SIU5 isolation unit, Grass Instruments, Quincy, MA) with pulse trains (1 ms pulse duration, 1–5 s train duration, 0.1 mA intensity, and 60–100 Hz pulse repetition rate).
Endoscopic Video Recording.
Syringeal movements were filmed by inserting an angioscope into the opening in the trachea and guiding the lens close to the syrinx. Exterior views (high vocal center experiments) were obtained by guiding the angioscope close to the syrinx from the ventral side after partly removing m. pectoralis, opening the sternum, retracting the heart, and resealing the insertion with tissue adhesive. The angioscope (Olympus, New Hyde Park, NY, type AF 14, 1.4-mm outer diameter, 75° field of view) was connected to an image control unit (Olympus, OTV-A), a 300 W Xenon light source (Olympus, type CLV-A), and a monitor (Olympus, type OEV-141).
Data Recording and Analysis.
Vocalizations monitored with a microphone (Sennheiser, Old Lyme, CT, MKH 416 TU-3), stimulation trains, and air sac pressure were recorded on a multichannel recorder (Metrum, Denver, CO, type RSR 512). For data analysis, recordings were digitized (Data Translation, Marlboro, MA, type DT-2821-G) with a sample rate of 40 kHz (playback at half speed) and analyzed with signal version 3.0 software (Engineering Design, Belmont, MA). Angioscope and microphone output were recorded on a videocassette recorder (Panasonic, type AG-W1-P, NTSC, 30 frames per s, shutter speed 1/50 s) with the sound channel being used for synchronizing video images with Metrum recordings. Video segments were digitized (Vincent PCI board and Media100 software, Data Translation) on a Power Macintosh 9500 computer and imported to software packages Photoshop version 3.0 or Corel version 5.0 for preparation of figures. Biomechanical aspects of the syrinx were verified by inspecting dissected specimens, and anatomical information was derived from microscopic frontal sections of a brown thrasher syrinx.
RESULTS
Despite full incapacitation of both MTM, zebra finches and cardinals readily vocalized. Although the vocalizations generally were produced at a lower intensity, calls and song remained nearly intact (Fig. 1). Notable acoustic changes include a slightly lower fundamental frequency in some syllables in the zebra finch and reduced intensity of high-frequency components as well as more prominent second harmonics in the cardinal. However, it is not clear whether these changes are a direct result of the elimination of the MTM, the possibly reduced tension on the medial labia, or other effects of the surgical procedure. This experiment clearly indicates, however, that the MTM are not necessary for sound production and thus are not the principal sound-generating structures in the songbird syrinx.
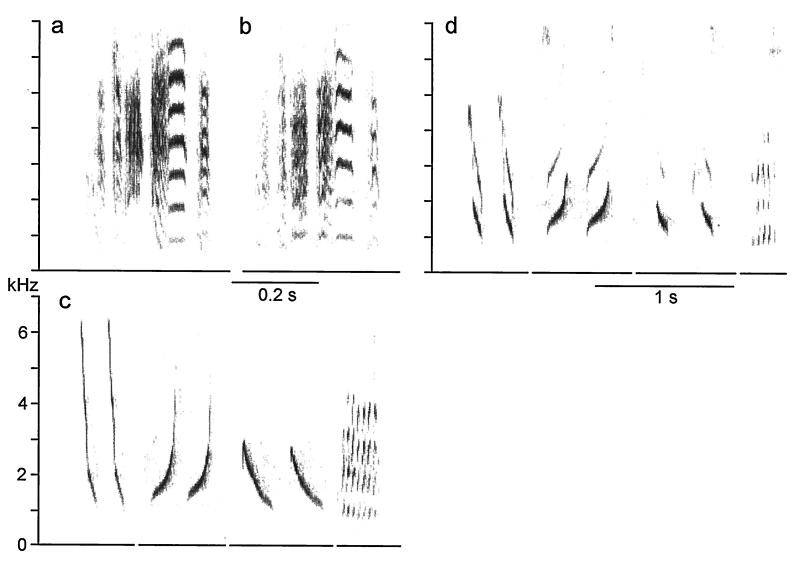
Figure 1.
Elimination of the MTM as a vibrating sound source does not mute birds and does not prevent them from producing nearly normal song. The song motif of a zebra finch remains intact after large holes were made into the left and right MTM, fully encompassing both. Song is shown spectrographically before (a) and 1 day after (b) the MTM were incapacitated as sound sources. Similarly, when both MTM were fully torn in a cardinal, preoperatively recorded syllable types (c) were sung nearly intact 1 day after the surgical removal of the MTM (d). In both species small changes in the acoustic fine structure of song, especially in harmonic emphasis, may be caused by the loss of the MTM, but surgical effects, such as fluid build-up and the sealing of the interclavicular air sac membrane with tissue adhesive, cannot be ruled out as possible causes.
The visual images of the vocal organ during sound production in three songbird species show the phonatory position of the syrinx (Fig. 2). In preparation for phonation, both sides of the syrinx undergo reconfiguration that is similar for spontaneous and brain-stimulated vocalizations in all three species (Figs. 3 and 4). The vocal organ is drawn craniad, probably by action of the tracheolateralis muscles. The result is that the free bronchial semi-rings of the syrinx are pulled apart along the rostro-caudal axis, the MTM and associated connective tissue are stretched, and suprasyringeal tracheal rings are drawn closer together. At the same time the ML and lateral labia (LL), which are loose connective tissue masses, are moved into the bronchial lumen, thereby forming a valve. LL is moved by rotation and mediad movement of the third bronchial semi-ring, whereas the cartilage tensor, a ventro-medial extension of the second bronchial semi-ring (10), may mediate movement of ML. In thrashers and cardinals, the labia also move craniad with respect to the semilunar membrane (SM) (Fig. 2 a and b), but in the crow the SM moves in concert with the labia. This difference can be attributed to the absence of an ossified pessulus and lack of structural support in the crow (15) (Figs. 2 a and c and 4). The excursion of the LL into the bronchial lumen is larger than that of the ML, and during full closure (adduction) it obstructs between about 60% and 85% of the lumen. These movements are caused by activity of syringeal muscles because low-intensity brain stimulation can reconfigure the syrinx without the generation of positive air sac pressure. Normally, however, build-up of subsyringeal pressure occurs simultaneously with syringeal reconfiguration and may contribute to the rostrad movement.
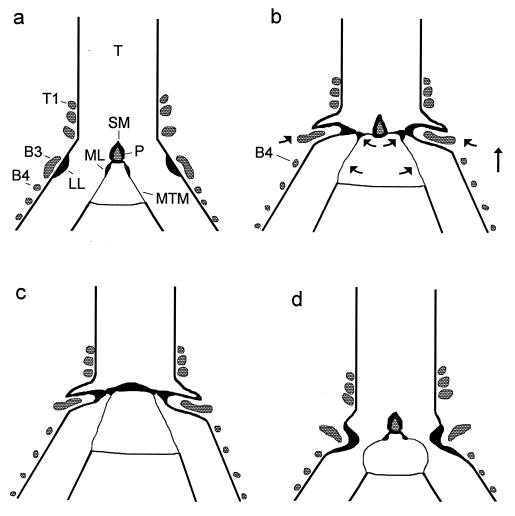
Figure 2.
(a) Configuration of the syrinx during quiet breathing in a frontal section (schematic). B3, B4, third and fourth bronchial ring; P, ossified pessulus; T, trachea; T1, first tracheal ring. (b) In the phonatory position the syrinx is moved rostrad and the bronchi are stretched (indicated by arrows), which, in concert with activity of the dorsal syringeal muscles, causes both labia to move mediad. LL is pushed into the lumen by B3, which presumably is rotated by dorsal muscle activity and also moved mediad by syringeal stretching. Movement of ML presumably is mediated by a thin, cartilaginous extension from the ventral end of B2 (not illustrated). (c) The dome-shaped phonatory position of the crow syrinx lacking an ossified pessulus. (d) The “classical model” of the phonatory position postulates constriction of the syrinx by the LL and vibrating MTM (redrawn after refs. 7 and 11).
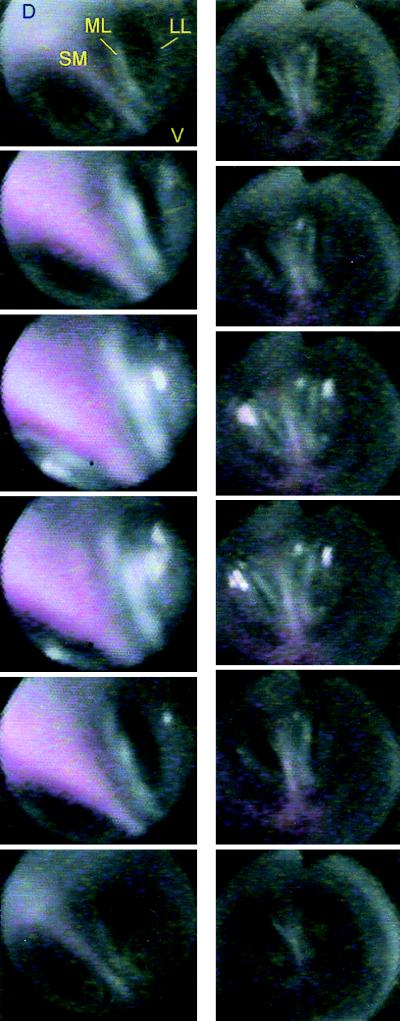
Figure 3.
Individual frames taken from video segments (duration about 1 s) illustrate syringeal configuration during the transition from quiet respiratory to phonatory position in brown thrasher (Left, Top to Bottom) and cardinal (Right). The vocal organ is viewed from the top with the angioscope inserted into the trachea. The syrinx moves rostrad (increasing size of syringeal structures because of decreasing distance to angioscope lens and increased brightness of light reflections), and the labia move simultaneously into the bronchial lumen, resulting in bilateral closure. Phonation occurred during frames three and four (Left and Right). Black triangular notch on images (Right, top of frames) stems from an indicator in the angioscope.
Figure 4.
During spontaneously emitted calls, the crow syrinx (viewed from top) undergoes a reconfiguration similar to that of the thrasher and the cardinal (frames show transition from quiet respiration to phonation). A soft call (left angioscope column, upper spectrogram, and rectified amplitude waveform, A) is generated with incomplete closure of the ventral portion (arrows) of both syringeal halves. In contrast, during a louder call (right angioscope column, lower spectrogram, and waveform) there is complete bilateral closure and a greater rostrad excursion (distance between angioscope lens and syrinx in frame 1 is greater on Right than that on Left) of the syrinx. High subsyringeal pressure inflates the bronchial septum such that the ML cannot be clearly separated from the SM. The absence of an ossified pessulus in crows (15) probably facilitates this deformation. Because of the longer duration of the loud call, the return of the syrinx into respiratory position is not shown (Right).
Brain stimulation-induced (thrasher and cardinal) as well as spontaneous (crow) phonation is never observed unless both sides of the syrinx are constricted simultaneously and the luminal edges of the ML and LL of each side make contact along the dorso-ventral axis. When sound is produced, the labial folds vibrate at low amplitude. However, the low temporal resolution of the video equipment does not allow detailed analysis of the vibratory motion. With the labia in full contact the MTM could not be observed from above, but observations of the external ventral surface of the syrinx from the interclavicular air sac do not show blurring of the MTM, which would result if large excursions took place during phonation.
The endoscopic recordings also reveal details about amplitude control. Low volume sounds are generated when contact between the LL and ML is restricted to the dorsal part of the syrinx where the apparent vibrations occur. The incompletely adducted ventral part probably allows air to escape unmodulated. Increasing sound intensity is accompanied by more complete closure of the syrinx, a larger craniad deflection of the labia, and increased subsyringeal pressure (Fig. 5). During high-intensity calls in the crow the labia of both sides of the syrinx and the SM form a dome projecting craniad into the trachea (Figs. 2c and 4). This general observation about sound intensity also applies to amplitude modulation (AM). In thrashers, vocalizations elicited by brain stimulation frequently are characterized by marked regularly repeated AM (oscillatory AM). This amplitude modulation is accompanied by slight retraction (abduction) of the ventral part of the LL, and possibly also of the ML, on the right, but not on the left, side of the syrinx. As seen in a syllable with 100% modulation (Fig. 5) even a slight opening of the airways is sufficient to reduce sound amplitude to zero, presumably by causing a significant amount of air to bypass the vibrating structures. This reduction in syringeal resistance also is reflected in a decrease in air sac pressure. Interestingly, oscillatory AM was found to be a common feature of spontaneously produced thrasher song and usually was generated by modulating airflow through the right side of the syrinx (31).
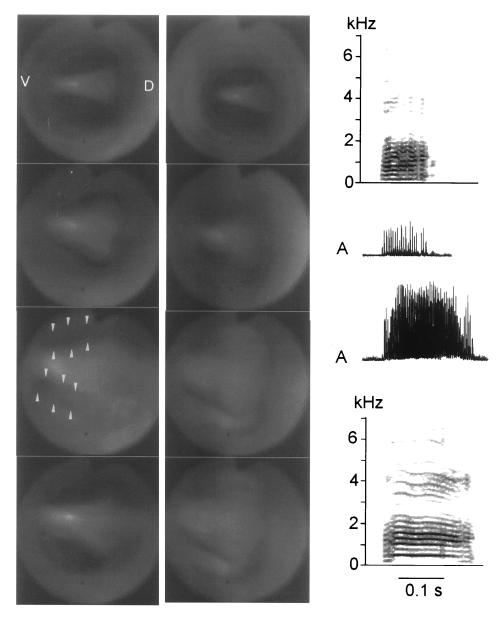
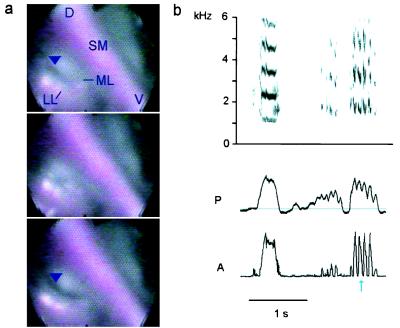
Figure 5.
Amplitude modulation of a brown thrasher vocalization is clearly visible as a correlated partial opening of the right valve formed by the adducted labia. (a) Illustration of the slight partial abduction (arrowhead) and fully closed position of the right side during one modulation cycle. On the left side the labia remain in close contact throughout the cycle (D, dorsal; V, ventral). (b) Series of representative vocalizations evoked by electrical brain stimulation is shown spectrographically (Upper) and as rectified sound amplitude (A) (Lower) together with the subsyringeal air sac pressure (P, horizontal line indicates ambient pressure). Sounds may be almost unmodulated (Left) or 100% amplitude modulated (Right). The blue arrow marks the modulation cycle illustrated in a.
DISCUSSION
With these direct images of the oscine syrinx during spontaneous and brain stimulation-induced vocalization, events leading to phonation can be described clearly (Fig. 2 a–c). The rostrad movement of the vocal organ exerts tension and thus stretches the elastic tissues, including the MTM, of the bronchial area and rearranges geometric relationships of the various cartilage components, which indirectly contribute to aperture control. This dynamic nature of the reconfiguration of the syrinx before and during vocalization illustrates clearly that many details of the complex mechanisms of sound generation in birds can be unraveled only by studying the intact vocal organ in situ.
The endoscopic observations and the observation that songbirds are able to sing without functional MTM have implications for the physical mechanism of sound generation, which are in marked contrast to previous models. Closure of the bronchial aperture by approximation of the ML and LL, with at least partial apposition along the dorso-ventral axis, is essential for sound production. Apparent full adduction and vibrations of the labia suggest that phonation may involve opening and closing motions of the luminal edges of both labia, presumably induced by Bernoulli forces. These observations also speak against a sound production mechanism via a hole tone whistle, in which the edges of the two labia might act as turbulence-inducing lips. However, without detailed data on labial vibratory motion, a whistle mechanism, as originally proposed to explain production of pure tone vocalizations in parrots and doves (32, 33), cannot be ruled out completely.
In any case, the MTM are not necessary for sound production, although they may have a role in the fine control of acoustic structure. Thus, the present model posits the labia as the principal sound source in the oscine syrinx in contrast to the classical model, which neither attributes any role to the ML nor considers either labium as a potential vibratory structure (Fig. 2d). Only one previous study (34) proposes a model derived from acoustic analysis of myhna (Gracula religiosa) imitation of human speech that includes some of the features of the present description. Because the phonatory position and apparent sound-generating mechanism is similar in all three investigated species, representing members of three songbird families, we suggest that this model is likely to be the basic mechanism of sound generation in all songbirds, and it also shows parallels to non-passerine species (30, 35, 36). Moreover, the observed phonatory configuration in songbirds may be analogous to that of the human vocal folds, suggesting that the mechanisms of sound generation also may be similar despite many differences in the detailed anatomy of the vocal organ in birds and humans (5).
Acknowledgments
We are grateful to Drs. R. A. Suthers and J. M. Wild for support and encouragement. J. M. Wild kindly performed the postmortem inspections of the syrinx, and Dr. S. L. Ball introduced us to high vocal center stimulation. We thank R. A. Suthers, J. M. Wild, C. L. Pytte, and two anonymous reviewers for comments on the manuscript and J. and L. Jesseph for generously lending us the videocassette recorder. This study was supported by the Austrian Programme for Advanced Research and Technology, the Danish National Research Foundation, the National Institutes of Health, and the National Science Foundation.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: MTM, medial tympaniform membranes; ML, medial labium; LL, lateral labium; SM, semilunar membrane.
References
- 1.Konishi M. Annu Rev Neurosci. 1985;8:125–170. doi: 10.1146/annurev.ne.08.030185.001013. [DOI] [PubMed] [Google Scholar]
- 2.Nottebohm F. J Comp Physiol A. 1996;179:149–156. doi: 10.1007/BF00222782. [DOI] [PubMed] [Google Scholar]
- 3.Marler P. Am Scientist. 1970;58:669–673. [PubMed] [Google Scholar]
- 4.Hauser M D. The Evolution of Communication. Cambridge, MA: MIT; 1996. [Google Scholar]
- 5.Fink B R. The Human Larynx: A Functional Study. New York: Raven; 1975. [Google Scholar]
- 6.Gaunt A S. In: Bird Respiration. Seller T J, editor. Vol. 1. Boca Raton, FL: CRC; 1987. pp. 71–94. [Google Scholar]
- 7.Brackenbury J H. In: Form and Function in Birds. King A S, McLelland J, editors. Vol. 4. New York: Academic; 1989. pp. 193–220. [Google Scholar]
- 8.Miskimen M. Auk. 1951;68:493–504. [Google Scholar]
- 9.Greenewalt C H. Bird Song: Acoustics and Physiology. Washington, DC: Smithsonian Institution Press; 1968. [Google Scholar]
- 10.Setterwall C G. Ph.D. thesis. University of Lund; 1901. [Google Scholar]
- 11.Chamberlain D R, Gross W B, Cornwell G W, Mosby H S. Auk. 1968;85:244–252. [Google Scholar]
- 12.Ames P L. Bull Peabody Mus Nat Hist (Yale Univ) 1971;37:1–194. [Google Scholar]
- 13.Warner R W. J Zool London. 1972;168:381–393. [Google Scholar]
- 14.Gaunt A S, Stein R C, Gaunt S L L. J Exp Zool. 1973;183:241–262. [Google Scholar]
- 15.King A S. In: Form and Function in Birds. King A S, McLelland J, editors. Vol. 4. New York: Academic; 1989. pp. 105–192. [Google Scholar]
- 16.Suthers R A. Nature (London) 1990;347:473–477. [Google Scholar]
- 17.Stein R C. Auk. 1968;85:229–243. [Google Scholar]
- 18.Dürrwang R. Ph.D. thesis. University of Basel; 1974. [Google Scholar]
- 19.Brackenbury J H. J Theor Biol. 1979;81:341–349. doi: 10.1016/0022-5193(79)90171-1. [DOI] [PubMed] [Google Scholar]
- 20.Casey R M, Gaunt A S. J Theor Biol. 1985;116:45–64. [Google Scholar]
- 21.Fletcher N H. J Theor Biol. 1988;135:455–481. [Google Scholar]
- 22.Fletcher N H. Comm Theor Biol. 1989;1:237–251. [Google Scholar]
- 23.Fletcher N H. Acoustic Systems in Biology. New York: Oxford Univ. Press; 1992. [Google Scholar]
- 24.Gross W B. Poultry Sci. 1964;43:1005–1008. [Google Scholar]
- 25.Gross W B. Poultry Sci. 1964;43:1143–1144. [Google Scholar]
- 26.Gottlieb G, Vandenbergh J G. J Exp Zool. 1968;168:307–326. doi: 10.1002/jez.1401680303. [DOI] [PubMed] [Google Scholar]
- 27.Gross W B. Avian Dis. 1979;23:1031–1036. [PubMed] [Google Scholar]
- 28.Gaunt A S, Gaunt S L L, Hector D H. Condor. 1976;78:208–223. [Google Scholar]
- 29.Gaunt A S, Gaunt S L L. J Morphol. 1977;152:1–19. doi: 10.1002/jmor.1051520102. [DOI] [PubMed] [Google Scholar]
- 30.Goller F, Larsen O N. J Exp Biol. 1997;200:2165–2176. doi: 10.1242/jeb.200.16.2165. [DOI] [PubMed] [Google Scholar]
- 31.Suthers R A, Goller F, Hartley R S. J Neurobiol. 1994;25:917–936. doi: 10.1002/neu.480250803. [DOI] [PubMed] [Google Scholar]
- 32.Nottebohm F. J Comp Physiol. 1976;108:157–170. [Google Scholar]
- 33.Gaunt A S, Gaunt S L L, Casey R M. Auk. 1982;99:474–494. [Google Scholar]
- 34.Klatt D H, Stefanski R A. J Acoust Soc Am. 1974;55:822–832. doi: 10.1121/1.1914607. [DOI] [PubMed] [Google Scholar]
- 35.Beebe W. Zoologica. 1925;6:195–227. [Google Scholar]
- 36.Paulsen K. Das Prinzip der Stimmbildung in der Wirbeltierreihe und beim Menschen. Frankfurt am Main: Akademische Verlagsgesellschaft; 1967. [Google Scholar]