Abstract
A number of neuroimaging findings have been interpreted as evidence that the left inferior frontal gyrus (IFG) subserves retrieval of semantic knowledge. We provide a fundamentally different interpretation, that it is not retrieval of semantic knowledge per se that is associated with left IFG activity but rather selection of information among competing alternatives from semantic memory. Selection demands were varied across three semantic tasks in a single group of subjects. Functional magnetic resonance imaging signal in overlapping regions of left IFG was dependent on selection demands in all three tasks. In addition, the degree of semantic processing was varied independently of selection demands in one of the tasks. The absence of left IFG activity for this comparison counters the argument that the effects of selection can be attributed solely to variations in degree of semantic retrieval. Our findings suggest that it is selection, not retrieval, of semantic knowledge that drives activity in the left IFG.
What parts of the brain subserve the retrieval of semantic knowledge? A number of recent neuroimaging studies, using a range of different tasks, implicate the left inferior frontal gyrus (IFG) (1–8). Despite the impressive convergence of localizations across tasks as disparate as verb generation, stem completion, and abstract/concrete judgments, the conclusion that semantic retrieval critically involves left IFG remains uncertain for two reasons. First, a smaller but still significant number of neuroimaging studies have failed to find left IFG activation during semantic retrieval tasks. For example, neither naming pictures nor verifying word associations consistently leads to left IFG activation, despite the prima facie involvement of semantic knowledge in both tasks (9, 10). Additionally, practice of a semantic retrieval task, even after just a single repetition, causes a marked decrease in left IFG activation (2, 8). Second, neuropsychological studies of patient populations have so far failed to demonstrate the necessity of left IFG for semantic retrieval. Instead, impairments of semantic knowledge are most associated with temporal lobe pathology (11–13).
The goal of this article is to propose and test an alternative interpretation of the activation of left IFG during semantic retrieval tasks, based on recent theorizing about the role of prefrontal cortex in nonsemantic domains. Cohen and Servan-Schreiber (14) argued that prefrontal cortex enables flexible and context-sensitive responses, particularly in tasks where a response other than the prepotent one must be selected. Kimberg and Farah (15) characterized the role of prefrontal cortex in cognition as mediating the selection of action by the weighting of information active in working memory. When the contents of working memory are not critical for action selection, because the action is prepotent, or when the contents of working memory overwhelming support one action, then demands on prefrontal cortex are low. Their model implies that demands on prefrontal cortex will be high in any task that requires selection among competing sources of information in working memory to guide a response.
Our conjecture is that the activation of the left IFG during semantic tasks is the result not of semantic retrieval per se, but of the need to select some relevant feature of semantic knowledge from a set of competing alternatives. A feature common to semantic retrieval tasks in which left IFG activation has been found is the necessity of selecting among competing sources of information to guide response. In generation tasks, subjects must select just one of many associations to the given noun [e.g., action (6, 7); color (5)]. In classification tasks, subjects must select a single dimension of the object’s meaning to attend to [e.g., concreteness (2)]. A feature common to tasks showing diminished or absent left IFG activation is a relatively reduced requirement for selecting among competing sources of information. Comparisons of global similarity, which are based on all available information and thus do not require selection, do not activate the left IFG (10). Repetition, which decreases the need for selection by increasing the prepotency of the response, also decreases left IFG activity in generation and classification tasks (2, 8).
We predicted that the degree of left IFG activity, as measured by functional magnetic resonance imaging (fMRI) during semantic retrieval, could be modulated by manipulating the selection demands of the semantic task. To test this hypothesis, we used three tasks that have previously been used to study semantic retrieval: two tasks (generation and classification) have previously been associated with left IFG activity (2, 4, 6, 7) and one task (comparison) has not (10). Each task was modified to include a High Selection condition and a Low Selection condition (Fig. 1); we predicted increased activity in the left IFG during the High Selection condition relative to the Low Selection condition during all three tasks. Furthermore, because a single group of subjects comprised the study group for all three tasks, anatomical colocalization could be interpreted unambiguously; we predicted that the regions differentially active during High Selection conditions would converge on the same location in the left IFG.
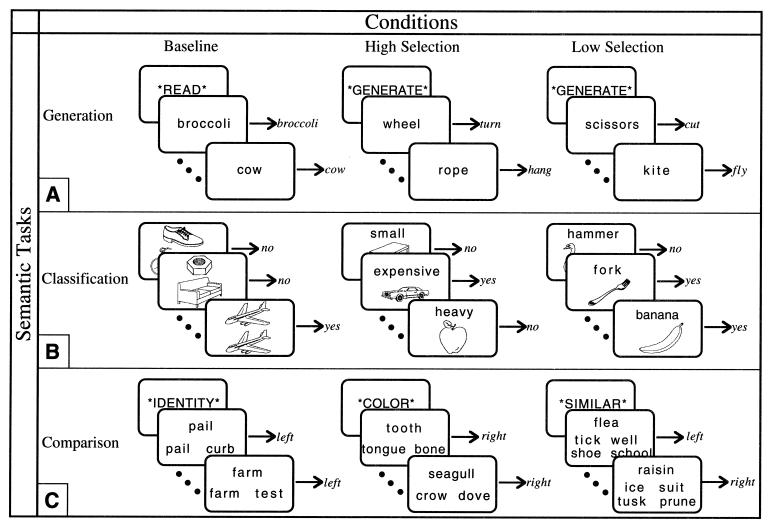
Figure 1.
High Selection, Low Selection, and nonsemantic Baseline conditions of each task were presented in a fixed-order blocked design, one condition per block alternating throughout each scan. For each task, two 12-block scans, separated by 3 min, were run. (A) During the Generation task, subjects either read a word silently [mean (M) response time = 631 ms] or silently generated a related verb. In each condition, subjects were required to indicate completion of the task with a bilateral button press. Each block contained an instruction for 400 ms, followed by 8 words, presented for 2,000 ms with a 400-ms interstimulus interval (ISI). For the Generate conditions, alternate blocks contained either High Selection (M response time = 1211 ms) or Low Selection (M response time = 1,041 ms) items. (B) During the Classification task, subjects classified line drawings of common objects (16). During the High Selection condition, subjects classified 10 pictures according to one of eight different attributes (big, small, light, heavy, manmade, natural, expensive, cheap), randomly presented within the block (M response time = 1188 ms, accuracy = 82%). During the Low Selection condition, subjects classified 10 pictures according to a basic level object name (e.g., spider, hammer) (M response time = 942 ms, accuracy = 99%). During the Baseline condition, subjects classified 10 pictures according to the physical identity of another picture (M response time = 726 ms, accuracy = 99%). On each trial, the stimuli were presented for 1,600 ms followed by a 400-ms ISI. For all items, subjects indicated a yes–no response by pressing one of two buttons. (C) During the Comparison task, subjects made comparisons between a target word and several probe words. In the Baseline condition subjects picked which of two words was the same as the target word (M response time = 664 ms). In the Low Selection condition subjects picked which of either two words (two-choice Low Selection, not pictured; M response time = 1,215 ms) or four words (four-choice Low Selection; M response time = 1,417 ms) was most similar in meaning to the target word. In the High Selection condition subjects picked which of two words was most similar along a specified dimension (color, function, or shape) to the target word (M response time = 1,463 ms); the dimensions were blocked so that in a given block attribute judgments were made about only one dimension but across the experiment there were two blocks of each dimension. Subjects indicated whether the correct probe was on the left or right of the target by pressing a corresponding button. (In the four-choice Low Selection condition, two words were presented on the left and two on the right; although there were four words, the choice was still binary.) Each block began with an instruction cue for 2,000 ms, followed by 9 words, presented for 1,800 ms with a 200 ms ISI.
MATERIALS AND METHODS
Behavioral Methods.
In the Generation task, subjects covertly generated a verb related to a visually presented noun. In the High Selection condition, items were nouns with many appropriate associated responses without any clearly dominant response; in the Low Selection condition, items were nouns with few associated responses or with a clear dominant response. The stimuli for the Generation task were 96 concrete nouns (Kucera–Francis frequency range from 0 to 591, median frequency = 32; word length range from 3 to 8, median = 4), divided into two groups on the basis of verb generation data from two independent groups of subjects (n = 30 and 50). Subjects were asked to generate a verb for each noun. A ratio of the relative frequency of the most common completion to the relative frequency of the second-most common completion was calculated as a measure of response strength. Nouns with a high response strength ratio (range from 5.00 to 50.0, median = 13.34) differed from nouns with a low response strength ratio (range from 1.0 to 3.0, median = 2.0) in terms of response strength ratio, t (94) = 13.85, P < 0.001, but not word frequency, t (94) = 0.76, P = 0.45 (statistics were performed on the cube-root of frequency and ratio). Ninety-six concrete nouns were selected as items for the baseline condition, matched to the items for the Generate conditions on the basis of word length, t (190) = 0.00, P = 1.0, and word frequency, t (190) = 0.44, P = 0.66.
In the Classification task, subjects classified line drawings of common objects. In the High Selection condition, pictures were classified according to one specific attribute of the object’s representation; in the Low Selection condition, pictures were classified according to basic level object names. Whereas in the High Selection condition, the task requires responding on the basis of a single attribute of the object’s representation, in the Low Selection condition the entire description or representation of the object is relevant to a name classification.
In the Comparison task, subjects compared a target word to several probe words and decided which probe was most similar. In the High Selection condition, comparisons between items were based on specific attributes or features; in the Low Selection condition, comparisons were based on global similarity. Two Low Selection conditions were used: in the two-choice Low Selection condition subjects compared two probes to the target, and in the four-choice Low Selection condition subjects compared four probes to the target.
These conditions were included for two reasons. First, the four-choice Low Selection condition was a better match for the High Selection condition in terms of response time. Response latency between the four conditions in the Comparison task was compared by using analysis of variance of the median response time across the five subjects for each item, F (3, 212) = 254.87, P < 0.001. Response times were fastest in the Nonsemantic condition, and were faster in the two-choice Low Selection condition than in the other Semantic conditions. However, there was no difference in response times between the four-choice Low Selection condition and the High Selection condition. This control was important because neuroimaging signal is sensitive to the duration of neural activity as indexed by response time (17).
Second, whereas the Low Selection and High Selection conditions differed in terms of selection demands, the two-choice Low Selection and four-choice Low Selection conditions differed only in terms of amount of semantic retrieval, with a maximum of two and four semantic comparisons per trial, respectively. This additional comparison allowed us to address directly the alternative hypothesis that left IFG activity reflects simply increased semantic processing.
Across all three tasks, in the High Selection condition, retrieval of semantic knowledge of an object made available some semantic information that was relevant to the task and other semantic information that had to be ignored, such as inappropriate associations or irrelevant features of the object. In the Low Selection condition, all of the available semantic information was relevant to the task, either in making a global similarity judgment, identifying the name of the object, or choosing the most dominant association. To the extent that left IFG activation reflects the process of selecting relevant semantic knowledge among other available but irrelevant knowledge, activation should be greater in the High Selection conditions than in the Low Selection conditions.
fMRI Methods.
Six whole-brain echoplanar fMRI scans were collected from five right-handed subjects. Following the acquisition of sagittal (TR = 500, TE = 11, 128 × 256, 1NEX) and axial (TR = 600, TE = 15, 192 × 256, 2NEX) T1 weighted localizer images, gradient echo, echoplanar fMRI was performed in 18 contiguous 5-mm axial slices (TR = 2,000, TE = 50, 64 × 64 pixels in a 24-cm field of view) using a 1.5-T GE signa system equipped with a prototype fast gradient system and the standard quadrature head coil. Twenty seconds of “dummy” gradient and rf pulses preceded the actual data acquisition to approach tissue steady-state magnetization. Head motion was minimized by using foam padding. A total of six 4-min scans were conducted for each subject, resulting in 720 observations per voxel per subject. Off-line data processing was performed on Sun Sparc workstations using programs written in Interactive Data Language (Research Systems, Boulder, CO). After image reconstruction, the data were sinc interpolated in time to correct for the fMRI acquisition sequence. A slicewise motion compensation method was utilized that removed spatially coherent signal changes by the application of a partial correlation method to each slice in time (18).
The raw data for each subject were transformed to a standardized spatial frame (19) by landmark-guided, nine-parameter differential scaling and spatially smoothed by convolution with a 5-voxel full width at half maximum Gaussian kernel. Spatial smoothing was undertaken to account for residual differences in anatomy after realignment. Voxelwise analysis was performed by using a general linear model for serially correlated error terms (20); included within the model was an estimate of intrinsic temporal autocorrelation (21), global signal covariates, and sine and cosine regressors for frequencies below that of the task. The global signals were not significantly correlated with the selection comparison for any of the tasks, t values < 0.5. Temporal data were smoothed with an empirically derived estimate of the hemodynamic response of the fMRI system (18). This analysis has been empirically demonstrated to hold the mapwise false positive rate at or below tabular values (20). Given values for effective degrees of freedom, smoothness, search volume, desired minimum cluster volume (14 voxels or 1 cm3), and desired α, we calculated a critical t value (22) of ≈3.7 for each map. The use of a cluster result (23) requires the assumption of a Gaussian field and seemed justified because of the large number of effective degrees of freedom (df = 307) of each study. Each subject’s statistical parametric map was thresholded at the critical t level and individual clusters below the minimum volume were removed.
RESULTS AND DISCUSSION
For each task, a direct comparison of High Selection and Low Selection conditions yielded several regions of significant differences (Table 1 and Fig. 2). Additionally, each condition was compared with the common baseline condition for that task. Results regarding left IFG were as predicted in all three tasks.
Table 1.
Local maxima of statistical maps of the High Selection condition compared to the Low Selection condition (and in the Comparison task, in the four-choice Low Selection condition compared to the two-choice Low Selection condition)
| Region | Comparison
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generation High–Low selection
|
Classification High–Low selection
|
High–Low selection
|
Four-choice– two-choice
|
|||||||||
| Tal. (x, y, z) | BA | t | Tal. (x, y, z) | BA | t | Tal. (x, y, z) | BA | t | Tal. (x, y, z) | BA | t | |
| Frontal | −49, 8, 30 | (L 44) | 3.98 | −38, 15, 30 | (L 44) | 10.39 | −45, 4, 30 | (L 44) | 5.30 | |||
| 38, 15, 23 | (R 44) | 4.34 | ||||||||||
| −41, 30, 8 | (L 45) | 4.68 | ||||||||||
| −38, 8, 34 | (L 9) | 10.63 | −4, 38, 34 | (L 8) | 3.88 | |||||||
| 41, 15, 34 | (R 9) | 5.38 | ||||||||||
| SMA/Cingulate | −4, 11, 45 | (M 6) | 4.59 | −4, 11, 53 | (M 6) | 7.84 | −4, 11, 53 | (M 6) | 4.99 | |||
| Temporal | −49, −53, 0 | (L 21) | 5.08 | |||||||||
| −38, −60, −15 | (L 37) | 4.52 | −49, −56, −8 | (L 37) | 4.64 | |||||||
| Parietal | −34, −68, 45 | (L 7) | 4.44 | −11, −68, 49 | (L 7) | 6.33 | ||||||
| 8, −71, 53 | (R 7) | 6.16 | ||||||||||
| Occipital | −30, −71, 34 | (L 19) | 7.72 | −19, −83, −4 | (L 18) | 6.00 | ||||||
| 23, −86, 11 | (R 19) | 5.88 | ||||||||||
Numbers in parentheses refer to Brodmann’s areas (BA). L, left; R, right, M, midline. Coordinates are expressed in millimeters in the Talairach and Tournoux brain atlas (18): x, medial–lateral axis (negative, left); y, anterior–posterior axis (negative, posterior); z, dorsal–ventral axis (negative, ventral).
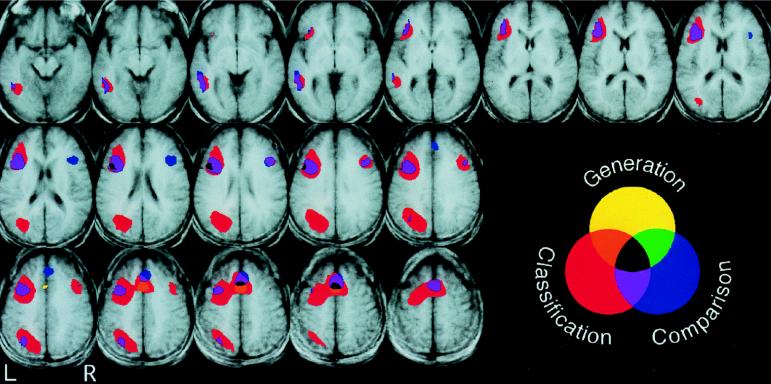
Figure 2.
Axial slices, 3.75 mm thick (z = −11 to z = 53), showing regions of significantly greater signal during the High Selection condition compared with the Low Selection condition in the Generation task (shown in yellow), the Classification task (shown in red), and the Comparison task (shown in blue). Overlap between pairs of tasks is shown in purple, green, and orange, and overlap between all three tasks is shown in black. Shown are voxels that exceeded a threshold of t = 3.67, mapwise α = 0.05 (corrected for multiple comparisons).
In the Generation task, left IFG activity was greater during both Generate conditions than during the Baseline condition. As predicted, left IFG activity was greater during the High Selection Generate condition than during the Low Selection Generate condition. The area of activity within the left IFG identified in the initial statistical parametric map surpassed the magnitude test but was below the cluster threshold. Thus, a region of interest approach was utilized to increase sensitivity within the left IFG by reducing the number of multiple comparisons for which correction was required. This approach seemed justified, as the left IFG was the a priori subject of the current study. The main effect of the Generate tasks was used to define a post-hoc region of interest in which the orthogonal contrast of High Selection versus Low Selection could be tested. The 100 voxels surrounding the point of maximum statistical difference between the two Generate tasks and the Baseline condition were identified and the average time series within this region was evaluated for the High vs. Low Selection comparison. The single time series was evaluated with an uncorrected α = 0.05, t (307) = 3.12, P < 0.001. In addition to the predicted differences in the left IFG, the High Selection condition also showed increased activity near the borders of the supplementary motor area (SMA) and anterior cingulate gyrus.
In the Classification task, only the High Selection condition showed significantly greater activation in the left IFG compared with the Baseline condition. The direct comparison of the High Selection and Low Selection conditions revealed the predicted differences in left IFG, in addition to differences in the SMA, anterior cingulate gyrus, and left fusiform gyrus.
In the Comparison task, there were two critical comparisons (Fig. 3). As with the other tasks, the direct comparison of the High Selection and Low Selection conditions revealed the predicted differences in the left IFG. Importantly, this difference was found between the High Selection condition and the four-choice Low Selection condition, which was matched with the High Selection condition in terms of response time.
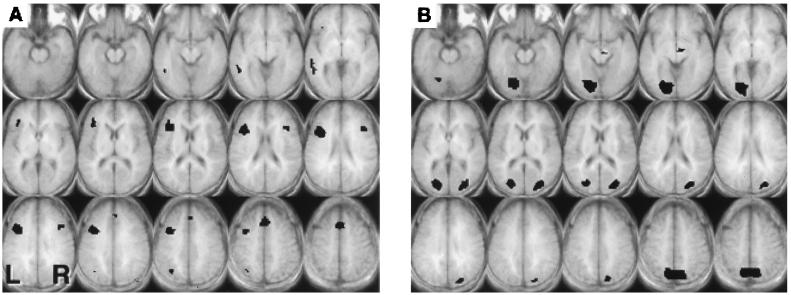
Figure 3.
Axial slices, 5 mm thick (z = −15 to z = 55), showing voxels that exceeded a threshold of t = 3.67, mapwise α = 0.05 (corrected for multiple comparisons) for the Comparison task. (A) Regions of significantly greater signal during the High Selection condition compared with the four-choice Low Selection condition. (B)) Regions of significantly greater signal during the four-choice Low Selection condition compared with the two-choice Low Selection condition.
As with the other tasks, this finding argues that selection demands influence left IFG activity. However, those findings cannot rule out the possibility that more semantic processing is happening in the High Selection conditions. If this alternative hypothesis provides the best account of these data, one would expect that a similar difference in left IFG activity would emerge in the comparison between the four-choice Low Selection condition and the two-choice Low Selection condition, because there are a greater number of required semantic comparisons in the four-choice condition. Instead, differences between the two Low Selection conditions were confined to posterior cortical regions (visual association cortex). To be certain that the absence of significant activation in left IFG in this critical comparison was not a result of our thresholding level, we used a less conservative analysis to determine if there was even a trend toward an increase in left IFG activity in the four-choice Low Selection condition. A region of interest analysis (similar to that described above for the generation tasks) with an uncorrected α = 0.10 yielded no differences in left IFG between the four-choice and two-choice Low Selection conditions, t < 0.5. Therefore, we can reject the hypothesis that mere increases in the amount or duration of semantic processing will be associated with increases in left IFG activity.
As already noted, a variety of different tasks, including those used in the current study, have been used to study semantic retrieval. In a review of eight semantic retrieval studies, Buckner and Tulving (24) suggest that the activations cluster at or near Brodmann’s areas 44 and 45, consistent with our findings. However, comparisons of the brain regions activated by these tasks have never been carried out in the same group of subjects. The fully within-subject design of this study allowed for a comparison of differences related to selection demands between the three types of semantic retrieval tasks. This design is similar in spirit to an alternative approach to structuring imaging experiments called “cognitive conjunction” that has reduced reliance on the assumptions of pure insertion to make inferences about functional localization compared with cognitive subtraction (25). All three semantic retrieval tasks showed effects of selection demands in overlapping regions in left IFG, with local maxima for the three tasks located in the same superior–inferior plane and within a few millimeters of each other. This pattern of results is consistent with our assertion that left IFG activity reflects the degree of selection among competing alternatives, and not the amount of semantic retrieval per se. The remarkable convergence across three disparate semantic retrieval tasks, which vary in terms of task demands, stimulus type (pictures and words), and response type (covert generation, binary decisions), argues for a general process or mechanism related to left IFG activity. It is this very diversity of setting in which the putative process of selection is evoked that results in a reduced vulnerability to failures of pure insertion specifically and cognitive subtraction in general.
As with all functional neuroimaging studies, these results do not enable us to make inferences about the necessity of left IFG to selection tasks (26). Instead, our inferences must be limited to a description of the association between selection demands and left IFG activity. The proposed distinction of semantic tasks according to selection demands is not one that has been made routinely in neuropsychological testing of patients with prefrontal lesions. Robinson and colleagues (27) reported that an aphasic patient with a meningioma impinging on left IFG was able to generate verbal responses only for stimuli with a few responses or a single prepotent response. Randolph and colleagues (28) found that providing retrieval cues on a verbal fluency task to a subject with ventral prefrontal damage dramatically improved the patient’s fluency performance, in contrast to Alzheimer’s disease patients with more widespread damage, who showed no improvement with cueing. Although it would be premature to conclude that these studies demonstrate that the left IFG is necessary for selection among competing responses, these findings are certainly consistent with that conjecture.
Although we had no a priori predictions about the effect of selection demands on other cortical regions, differential activation during the High Selection conditions of all three tasks was also observed near the border of the anterior SMA and the anterior cingulate gyrus. In addition to left IFG activation, during a verb generation task differential activation was also found in the anterior cingulate (6, 7) and SMA (10). The anterior cingulate has previously been associated with selective attention, attention-to-action, and executive functions (29–31). Likewise, the anterior region of the SMA, or pre-SMA, is associated with complex tasks across a variety of domains (32). Both the anterior cingulate and pre-SMA have strong, reciprocal connections with the prefrontal cortex (33, 34). Whether or not the functions of this medial area and the left IFG differ in any significant way would have to be addressed in future studies.
In two of the three tasks, increased activity during the High Selection conditions was observed in the left fusiform gyrus. Previous studies have found left fusiform activity during tasks that require mental imagery or retrieval of visual knowledge (35–37). The two semantic retrieval tasks showing differences in this region were the Comparison and Classification tasks. In the Classification task, two of the attributes used to classify the pictures in the High Selection condition tapped visual knowledge of size; in the Comparison task, two of the three comparisons in the High Selection condition tapped visual knowledge of color or shape. Because specific visual knowledge was required for a considerable proportion of the High Selection trials in these two tasks, activity in the fusiform gyrus was perhaps expected. In the current study it was not possible to analyze these trials separately; thus, we cannot, of course, distinguish between this proposed cognitive process and selection. However, we would predict that a design that allows such an analysis would find that fusiform activity was related to the type of knowledge being retrieved and not to the degree of selection demands.
The hypothesis that left IFG activity is driven by a general mechanism of selection was based on the conjecture that prefrontal cortex mediates selection in a wide range of both semantic and nonsemantic tasks [e.g., Stroop task (38, 39)]. In the current study we chose to address only the role of selection in semantic retrieval and therefore confined our tasks to those that tapped semantic knowledge. As a result, we cannot conclude whether the convergent region of activation in left IFG observed across these tasks under High Selection conditions is related to the selection of available semantic information specifically, or whether the selection of any type of response would be associated with left IFG activity in this particular region. One possibility is that this region of left IFG subserves a general response selection mechanism that is used across a variety of tasks and domains. A second hypothesis, suggested by Goldman-Rakic (40), is that different subregions of prefrontal cortex may perform similar operations on different input information. According to this hypothesis, the region of the left IFG identified in the current study may be specialized for the selection of semantic information but not other types of information.
Several other hypotheses about the role of the left IFG have been proposed on the basis of functional neuroimaging data. Many authors (2, 4, 6, 7, 41) have concluded that the left IFG mediates the retrieval of semantic knowledge. However, in the current study the left IFG was modulated across tasks that all required semantic knowledge. Furthermore, a specific increase in the total duration of semantic retrieval activity (as indexed by response time) in the Comparison task yielded no changes in left IFG activation. An increase would be expected with this experimental design if, indeed, semantic retrieval alone involves the left IFG. Frith and colleagues (42) argued that left IFG activation was the result of intrinsic generation of a response. In the Comparison and Classification tasks, where the response was fully specified by the stimulus (as opposed to a response requiring generation), left IFG activity was nonetheless present in the High Selection conditions. Shallice (43) proposed that the prefrontal cortex is necessary for the performance of novel tasks, which was supported by evidence that left IFG activity decreases with repetition (2, 8). However, the reduction or elimination of left IFG activation in the Low Selection conditions of the three tasks used in the current study occurred under novel conditions. In sum, none of the alternative hypotheses for the role of the left IFG can adequately account for either previous reports or the present findings. Instead, our results are consistent with the hypothesis that the left IFG is involved in the selection of some aspect or subset of available information among competing alternatives. We suggest that this mechanism is part of a more general prefrontal system that is required for the selection of responses from memory.
Acknowledgments
We thank Julie Fiez for providing us with normative data from the verb generation task and John Gabrieli, Jonathan Cohen, Edward Smith, and an anonymous reviewer for helpful comments on an earlier version of this manuscript. This research was supported by the James S. McDonnell Foundation and by National Institutes of Health Grants R01 NS34030, R01 AG14082, K02 AG00756, NS01762, and AG13483.
ABBREVIATIONS
- fMRI
functional magnetic resonance imaging
- IFG
inferior frontal gyrus
- SMA
supplementary motor area
References
- 1.Buckner R L, Petersen S E, Ojemann J G, Miezin F M, Squire L R, Raichle M E. J Neurosci. 1995;15:12–29. doi: 10.1523/JNEUROSCI.15-01-00012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demb J B, Desmond J E, Wagner A D, Vaidya C J, Glover G H, Gabrieli J D E. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Démonet J, Chollet F, Ramsay S, Cardebat D, Nespoulous J, Wise R, Rascol A, Frackowiak R. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 4.Kapur S, Craik F I M, Tulving E, Wilson A A, Houle S, Brown G M. Proc Natl Acad Sci USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin A, Haxby J V, Lalonde F M, Wiggs C L, Ungerleider L G. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- 6.Petersen S E, Fox P T, Posner M I, Mintun M A, Raichle M E. J Cognit Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- 7.Petersen S E, Fox P T, Snyder A Z, Raichle M E. Science. 1990;249:1041–1044. doi: 10.1126/science.2396097. [DOI] [PubMed] [Google Scholar]
- 8.Raichle M E, Fiez J A, Videen T O, MacLeod A K, Pardo J V, Fox P T, Petersen S E. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- 9.Martin A, Wiggs C L, Ungerleider L G, Haxby J V. Nature (London) 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- 10.Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Brain. 1991;114:1803–1817. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- 11.Hodges J R, Patterson K, Oxbury S, Funnell E. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- 12.Hodges J R, Graham N, Patterson K. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- 13.Saffran E M, Schwartz M F. In: Conscious and Nonconscious Information Processing: Attention and Performance XV. Umilta C, Moscovitch M, editors. Cambridge, MA: MIT Press; 1994. pp. 507–534. [Google Scholar]
- 14.Cohen J D, Servan-Schreiber D. Psychol Rev. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Kimberg D Y, Farah M J. J Exp Psychol General. 1993;112:411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- 16.Snodgrass J G, Vanderwart M. J Exp Psychol Hum Learn Mem. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 17.D’Esposito M, Zarahn E, Aguirre G K, Shin R, Auerbach P, Detre J A. NeuroImage. 1997;6:113–121. doi: 10.1006/nimg.1997.0281. [DOI] [PubMed] [Google Scholar]
- 18.Zarahn E, Aguirre G K, D’Esposito M. NeuroImage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]
- 19.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 20.Worsley K J, Friston K J. NeuroImage. 1995;2:173–182. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre G K, Zarahn E, D’Esposito M. NeuroImage. 1997;5:199–212. doi: 10.1006/nimg.1997.0264. [DOI] [PubMed] [Google Scholar]
- 22.Worsley K J. Adv Appl Probab. 1994;26:13–42. [Google Scholar]
- 23.Friston K J, Worsley K J, Frackowiak R S J, Mazziotta J, Evans A C. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 24.Buckner R L, Tulving E. Handb Neuropsychol. 1995;10:439–446. [Google Scholar]
- 25.Price C J, Friston K J. NeuroImage. 1997;5:261–270. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- 26.Sarter M, Berntson G G, Cacioppo J T. Am Psychol. 1996;51:13–21. doi: 10.1037//0003-066x.51.1.13. [DOI] [PubMed] [Google Scholar]
- 27.Robinson, G., Blair, J. & Cipolotti, L., Brain, in press.
- 28.Randolph C, Braun A R, Goldberg T E, Chase T N. Neuropsychology. 1993;7:82–88. [Google Scholar]
- 29.Pardo J V, Pardo P J, Janer K W, Raichle M E. Proc Natl Acad Sci USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posner M I, Petersen S E. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 31.Vogt B A, Finch D M, Olson C R. Cereb Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 32.Picard N, Strick P L. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- 33.Bates J F, Goldman-Rakic P S. J Comp Neurol. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- 34.Goldman-Rakic P S. In: Handbook of Physiology: Nervous System Vol V: Higher Functions of the Brain. Plum F, editor. Bethesda, MD: Am. Psychol. Soc.; 1987. pp. 374–417. [Google Scholar]
- 35.D’Esposito M, Detre J A, Aguirre G K, Alsop D C, Tippett L J, Farah M J. Neuropsychologia. 1997;35:725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- 36.Farah M J. Neuropsychologia. 1995;33:1455–1471. doi: 10.1016/0028-3932(95)00075-e. [DOI] [PubMed] [Google Scholar]
- 37.Thompson-Schill S L, Aguirre G K, D’Esposito M, Farah M J. Cognit Neurosci Soc Abstr. 1997;4:83. [Google Scholar]
- 38.George M S, Ketter T A, Parekh P I, Rosinsky N, Ring H, Casey B J, Trimble M R, Horwitz B, Herscovitch P, Post S M. Hum Brain Mapp. 1994;1:194–209. doi: 10.1002/hbm.460010305. [DOI] [PubMed] [Google Scholar]
- 39.Perret E. Neuropsychologia. 1974;12:323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- 40.Goldman-Rakic P S. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 41.Fiez J A. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- 42.Frith C D, Friston K J, Liddle P F, Frackowiak R S J. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- 43.Shallice T. From Neuropsychology to Mental Structure. New York: Cambridge Univ. Press; 1988. [Google Scholar]