Abstract
Multiple brain maps are commonly found in virtually every vertebrate sensory system. Although their functional significance is generally relatively little understood, they seem to specialize in processing distinct sensory parameters. Nevertheless, to yield the stimulus features that ultimately elicit the adaptive behavior, it appears that information streams have to be combined across maps. Results from current lesion experiments in the electrosensory system, however, suggest an alternative possibility. Inactivations of different maps of the first-order electrosensory nucleus in electric fish, the electrosensory lateral line lobe, resulted in markedly different behavioral deficits. The centromedial map is both necessary and sufficient for a particular electrolocation behavior, the jamming avoidance response, whereas it does not affect the communicative response to external electric signals. Conversely, the lateral map does not affect the jamming avoidance response but is necessary and sufficient to evoke communication behavior. Because the premotor pathways controlling the two behaviors in these fish appear to be separated as well, this system illustrates that sensory–motor control of different behaviors can occur in strictly segregated channels from the sensory input of the brain all through to its motor output. This might reflect an early evolutionary stage where multiplication of brain maps can satisfy the demand on processing a wider range of sensory signals ensuing from an enlarged behavioral repertoire, and bridging across maps is not yet required.
Segregated processing of both sensory and motor functions occurs extensively within the central nervous system. The separate processing streams are often channeled in multiple brain maps, each composed of populations of neurons with different physiological characteristics (1–5). However, at some points in the sensory–motor command chain, information processing spans across maps to yield the coherent percepts needed to recognize particular higher-order stimulus features or organize the multitude of motor components into an adaptive behavior (4–9). The 32 visual areas in macaques, for instance, are interconnected by more than 300 cortico-cortical pathways (6), and the up to 50 nuclei of the mammalian auditory brainstem are linked by a countless number of converging, diverging, and commissural connections (3). What is the functional and behavioral significance of these multiple sensory brain maps? In the visual system of cats and primates, for instance, three separate processing streams extend from the retina up to the cortex, each carrying different behaviorally low-level stimulus variables, such as different spatial and temporal frequency contents of stimuli (4, 6). In another prominent example, the two auditory subsystems in owls process sound intensity and phase information independently (7, 8). In each of these cases, however, the initial segregation of information processing is abandoned and information streams converge at higher-order levels in the brain yielding the computation of specific and behaviorally relevant stimulus features (3–9). This “distributed hierarchical” organization of sensory systems in higher vertebrates is inherently linked to the combinatorial nature of most complex sensory features extracted by these systems. But is this a general organizational principle that applies to all sensory systems?
The existence of very obvious multiple sensory maps with identical receptor inputs and mirror image boundaries in the first-order nucleus of the electrosensory system of gymnotiform fish, the electrosensory lateral line lobe (ELL), has always been a mystery, in particular, because all maps display the same cytoarchitecture and only show apparent differences in their overall size and the relative abundance of one cell type (9, 10). For electrolocation and communication purposes, these fish monitor the electric field that is produced by discharges of an electric organ with electroreceptors that are distributed over the body surface. Two distinct types of electroreceptors exist: low-frequency ampullary and high-frequency tuberous receptors (11). All electroreceptor afferents terminate in a somatotopic manner in the ELL of the hindbrain, where the rostral ELL represents the fish’s head region. The ELL consists of four mediolaterally adjacent segments: The medial segment receives input from ampullary afferent axons, whereas tuberous primary afferents trifurcate and each collateral innervates the three remaining segments, the centromedial (CMS), centrolateral (CLS), and lateral segment (LS) (9–11). No intermap connections have been found (12). Despite the lack of qualitative differences between the three tuberous maps, previous investigations yielded some quantitative differences in their physiological and immunohistochemical properties (13–15). Their behavioral significance, however, remained unclear.
Each segment of the ELL is highly laminated. The somata of large pyramidal cells are situated in a central layer. Pyramidal cells are the target of tuberous electroreceptor afferents. They have been shown to extract behaviorally relevant temporal features of modulations in electric field amplitude (16, 17). Pyramidal cells transmit this information to higher-order levels of the electrosensory system, particularly to the Torus semicircularis dorsalis in the midbrain, which is homologous to the inferior colliculus. Dorsal to the ELL pyramidal cell layer is the molecular layer that contains the large apical dendrites of pyramidal cells and is the site where extensive feedback through descending recurrent loops occurs (9, 10). Both N-methyl-d-aspartate and Kainic acid receptors are present in the molecular layer, and mainly N-methyl-d-aspartate-type receptors are present in the pyramidal cell layer of all four ELL segments (18, 19).
To determine their behavioral significance, we selectively lesioned each of the three tuberous ELL segments and monitored the neuronal activity at the injection site and the effects on two distinct behaviors of gymnotiform electric fish. Both sister groups (20), the knife fish Eigenmannia virescens and the brown ghost fish Apteronotus leptorhynchus, produce continuous nearly sinusoidal electric organ discharges (EODs) and the electrosensory pathways in these two taxa appear to be virtually identical (9). Eigenmannia very reliably lowers its own EOD frequency in response to a jamming signal of slightly higher frequency and raises its EOD frequency in response to a signal of slightly lower frequency. This jamming avoidance response (JAR; ref. 21), can provide a separation of EOD frequencies among neighboring fish that is required for accurate electrolocation of objects (9). Conversely, the JAR in Apteronotus is weaker and habituates quickly, and the fish can increase its frequency only in response to jamming signals (21). These differences in the behavioral patterns of the two fish appear to be based on differences in the premotor and not the electrosensory circuitry (22). During courtship and aggression, on the other hand, gymnotiform electric fish modulate their EODs, producing characteristic chirp-like signals. In Apteronotus (but rarely in Eigenmannia), chirping can be readily evoked experimentally by presenting an artificial electric signal of slightly different frequency from and with comparable amplitude as the fish’s own EOD (23, 24).
METHODS
Twenty-two specimens of Eigenmannia virescens and 10 Apteronotus leptorhynchus were used. All experiments were approved by the University of California Animal Care Committee and are in accordance with National Institutes of Health guidelines for experiments involving vertebrate animals. Fish were immobilized (1–2 μl of Flaxedil intramuscular, 2% for Eigenmannia and 20% for Apteronotus) and placed in a multicompartment chamber where up to four different body surface areas of the fish could be exposed separately to external signals. This was necessary because the high degree of convergence in the electrosensory system makes it highly resistant to physical trauma (9): when the whole body surface is exposed to external electric signals, the JAR, for instance, survives the loss of even very extensive lesions of the ELL. Hence, to minimize the consequences of convergent receptor input to the ELL, we limited the amount of body surface that was exposed to external electric signals. The electrical isolation between adjacent compartments was about 40 dB (25). A sinusoidal EOD replacement signal was delivered to each compartment through a pin electrode inserted in the dorsal musculature and an electrode at the bottom of the compartment. A sinusoidal external signal was presented through electrodes straddling the fish in each compartment. The two stimuli to each compartment could be individually delivered or removed by means of a switch board located outside the chamber. To elicit a JAR in Eigenmannia, the external signal was presented at frequencies alternating between 2 and 6 Hz above and below that of the EOD replacement, respectively, and delivered either in all compartments or only in two or three trunk compartments (compartments 3 and 4 or 2 to 4), excluding the head compartment. The relative amplitude ratio between jamming signal and EOD replacement signal was varied between 0.01 and 0.03. All stimulus parameters were always adjusted for maximum JAR performance.
To evoke chirping in Apteronotus, we delivered the external stimulus in the caudal compartments (compartments 2–4) at a fixed frequency 5 to 15 Hz above the fish’s own EOD frequency and with an amplitude of 1–2 mV/cm (23, 24). The stimulus was turned on only for a short period of time (1–3 s) at 1- to 2-min intervals to avoid habituation. Because Apteronotus has no myogenic but an electrogenic electric organ, its EOD is not attenuated in immobilized specimens, and hence no EOD replacement signal had to be presented. To determine both the somatotopic representation of the four chambers in the ELL segment of interest and the location of the pyramidal cell layer, we mapped the area by using single unit recordings of pyramidal cells. Search stimuli were presented only through the side electrodes of each compartment. For the three tuberous segments (CMS, CLS, and LS), it consisted of sinusoidal amplitude modulations between 2 and 10 Hz of an external stimulus with a carrier frequency similar to the animal’s own EOD frequency.
Portions of the ELL segment of interest were lesioned by pressure injecting 10 mM biotinylated ibotenic acid (26) into the pyramidal cell layer or the molecular layer. Ibotenic acid is an excitotoxic glutamate agonist that selectively affects only cell bodies and dendrites leaving fibers of passage intact (27). However, in our experimental paradigm, the survival times of the fish were usually not long enough to allow us to unequivocally verify the size of the lesion histologically because the extent of the gliosis becomes fully apparent only several days after injection of ibotenic acid. Thus, we conjugated ibotenic acid with biotin and used histochemical procedures to visualize the biotinylated ibotenic acid. The effects of biotinylated ibotenic acid did not differ from its unbiotinylated form (26). The effects of the drug injection were assessed by both recording neuronal responses to search stimuli at the injection site and monitoring the behavior. As a control, we injected similar amounts of carrier solution (Hepes) containing biotin and glycin at the usual concentrations but no ibotenic acid was injected into the CMS. At the conclusion of the control experiment, the electrode tract was marked by injecting current through the same barrel (17, 28). After a survival time of 3–12 h, the animal was perfused, and the brain was removed, sectioned on a Vibratome, and processed histochemically to visualize the biotinylated ibotenic acid by using a standard ABC/3,3′-diaminobenzidine reaction protocol (22, 26). Sections were analyzed light-microscopically by following the common nomenclature (29). EODs were amplified with a custom-made differential DC amplifier and stored on a video tape by using a PCM recording adapter (Vetter 3000A; sample rate 50 kHz). For subsequent analysis, signals were A/D-converted by using a commercial data analysis system (Signal, Engineering Design, Belmont, MA). To characterize the JAR, peak values of the EOD spectrogram were determined (sample rate, 10 kHz), and for the chirp analysis, the zero crossings of the EOD were calculated (sample rate, 25 kHz) and smoothed (running average, bin width 5 ms at 25 kHz). As a measure for the JAR, we used the maximum EOD frequency change during each JAR cycle. Chirps were characterized by their duration (measured in a frequency–time plot 50% above baseline) and the maximum frequency change. Statistical data analysis was performed by using commercial software (sigmastat/sigmaplot, Jandel, San Rafael, CA). Data were normalized relative to the mean (normally distributed) or median (nonnormally distributed) prelesion results for each experiment. Differences between pre- and postlesion data sets were compared by using t tests (normally distributed data) or Mann–Whitney rank sum tests (nonnormally distributed data).
RESULTS
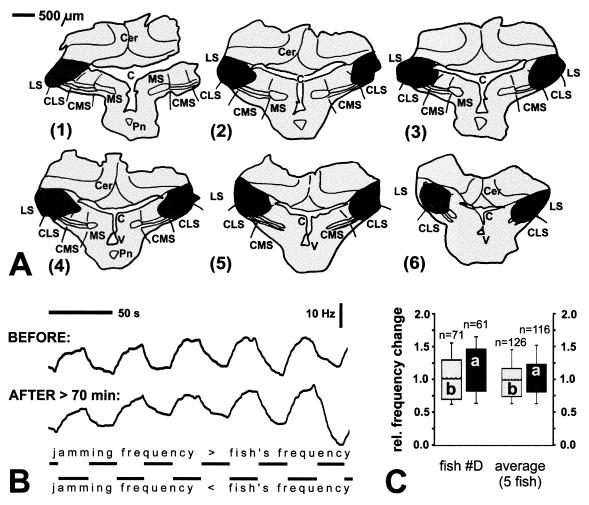
Inactivation of the various ELL segments had markedly different effects on the two behaviors tested. Lesions in the caudal part of the CMS in Eigenmannia, covering less than one-third of its total volume (Fig. 1A) reduced the JAR by more than 80% of its prelesion value when jamming signals were presented in the posterior compartments (parts 2–4) of the stimulation chamber (Fig. 1 B and C). Single and multiunit recordings in the injection area performed about 10 min after the injection revealed highly elevated neuronal activity of pyramidal cells that was, however, no longer correlated with the stimulus presented (Fig. 1D, center traces). Approximately 20 min later, any pyramidal cell activity at the injection site had ceased. In contrast, spherical cells, which are situated about 200 μm ventral to the pyramidal cell layer and exhibit no N-methyl-d-aspartate binding (19, 20), were not affected by ibotenic acid and still responded in a correlated manner (data not shown). Similarly, the responses of pyramidal cells located only about 500 μm lateral to the injection center were unchanged (Fig. 1D, bottom traces). In addition, when a jamming signal was presented in all compartments the JAR was also not altered significantly (Fig. 1 C and D). On average, after CMS lesions, the JAR reached only 16% of the prelesion value (Fig. 1C). Control injections of similar amounts of carrier solution did not affect the JAR (data not shown; rank sum test, P = 0.973).
Figure 1.
Lesion in CMS of Eigenmannia and effects on JAR. (A) Frontal sections (right half) through ELL, arranged from caudal (part 1) to rostral (part 6). Solid, center of injection; dark shading, periphery of injection (containing somata of pyramidal cells and dendrites reaching into center of injection); small solid circles, labeled somata of pyramidal cells. Sections are approximately 200 μm apart. MS, CMS, CLS, LS, medial, centromedial, centrolateral, lateral segments, respectively; Cer, cerebellum; Pn, pacemaker nucleus; V, fourth ventricle; C, cerebello-medullary cistern. (B) JAR before (upper traces) and after (lower traces) injection of biotinylated ibotenic acid as shown in A. Each pair of traces represents frequency–time plots of the EOD frequency. The upper trace in each pair shows the JAR for presentation of the jamming signal only in the caudal three compartments of the four-compartment chamber, and the lower one shows the JAR for presentation in all compartments (which had the same results as presenting signals only in the head compartment). Rows of horizontal bars indicate when the jamming frequency was higher (upper rows) or lower (lower rows) than the frequency of the fish’s EOD mimic. (C) Quantitative analysis of JAR before (bars b, light shading) and after (bars a, dark shading) the lesion. Data are the mean ± SD (n = number of JAR cycles). The asterisk shows significant differences (t test, P < 0.0001). First pair of boxes, JAR for signals limited to compartments 2–4 (as in A and B); second pair of boxes, JAR for signals presented in all compartments (as in A and B; no significant difference, t test, P = 0.304); last pair of boxes, mean JAR in five fish for signals limited to compartments 2–4. (D) Single unit recordings from different pyramidal cells before (upper pair of traces; on-response), shortly after injection of ibotenic acid, and about 500 μm lateral to injection center (outside injection area; off-response). The lower traces of each pair indicate the amplitude modulation of the stimulus presented. Recordings at 11 min and 32 min and the control recording were acquired with the same recording electrode.
In contrast to CMS lesions, inactivations of the other two tuberous segments, CLS and LS, clearly failed to affect the JAR (Fig. 2), despite the fact that the activity of pyramidal cells at the injection site underwent the same changes seen after injections in CMS (see Fig. 1D). Even after extensive lesions covering almost the entire CLS and LS bilaterally, such as the case depicted in Fig. 2, the postlesion JAR did not differ significantly from the JAR prior to the lesion (Fig. 2 B and C). Because there was no statistical difference between the results from unilateral and bilateral lesions (three and two fish, respectively; rank sum test, P = 0.932), we pooled all data for the calculation of the average JAR performance (Fig. 2C). We find that, on average, LS/CLS lesions had no effect on the JAR (Fig. 2C). It is noteworthy that also in two specimens of Apteronotus, which showed a relatively consistent JAR, CMS lesions but not lesions in the LS reduced the JAR (data not shown).
Figure 2.
Bilateral lesion in CLS and LS and significance for JAR in Eigenmannia. Same convention as in Fig. 1 A–C applies. (A) Frontal sections through ELL. Sections are about 250 μm apart. (B) Frequency–time courses of EOD for presentation of signals in compartments 2–4. (C) Medians of EOD frequency changes before and after lesion for same case depicted in A and B (left pair of boxes, rank sum test, P = 0.325) and average of five fish (right pair, rank sum test, P = 0.645). Lower and upper end of boxes define 25th and 75th percentile, respectively, with a solid horizontal line at the median and a hatched line at the mean. Error bars indicate the 10th (lower) and 90th (upper) percentiles.
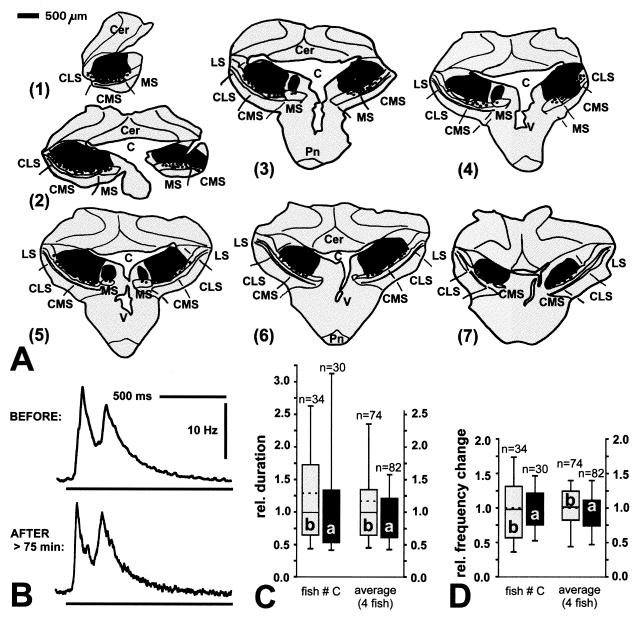
Unilateral lesions of portions of the LS, however, very consistently reduced the chirp response to externally presented electric signals in Apteronotus (Fig. 3). Approximately 30 min after LS lesions, evoked chirps that were by more than 80% shorter in their duration (Fig. 3C) and covered a 95% smaller frequency range than before (Fig. 3D). This is consistent with observations in untreated animals that a lower stimulus amplitude or a smaller body surface area exposed to external signals resulted in similar changes in evoked chirping behavior (data not shown; see also ref. 17). Yet, after lesions of the CMS and CLS, evoked chirping remained unchanged (Fig. 4). Even injections that covered almost the entire CMS and CLS bilaterally (Fig. 4A) did not affect either duration or EOD frequency change of evoked chirps (Fig. 4 C and D). Interestingly, two specimens of Eigenmannia also continued to produce intermittent chirps after lesions of the CMS, especially in response to signals presented at frequencies 5–10 Hz above the fish’s EOD simulation (data not shown) but no fish did so after lesions of the LS (n = 5).
Figure 3.
Lesion in LS and effects on evoked chirping in Apteronotus. Same convention as in Figs. 1 and 2 was used. (A) Frontal sections through ELL. Sections are about 250 μm apart. (B) Frequency–time plot of evoked chirp before (upper trace) and after (lower trace) injection of ibotenic acid. Horizontal bars indicate duration of external stimulus. (C) Quantitative analysis of effects on the duration of chirps. Left pair of boxes, examples taken from same case depicted in A and B; right box pair, average of three fish; asterisks, significantly different (rank sum test, P < 0.0001). (D) Effects on frequency change during evoked chirping. Same convention as in C.
Figure 4.
Bilateral lesion in CMS and CLS and significance for evoked chirp response in Apteronotus. Same convention as in Figs. 1 and 3. (A) Frontal sections, about 250 μm apart. Sectioning occurred asymmetrically. Thus, in part 1, only the right half of the brain was sectioned. (B) Examples of evoked chirps before (upper trace) and after (lower trace) injection of ibotenic acid. (C) Effects on relative duration of evoked chirps. Left pair of boxes, same fish as shown in A and B (rank sum test, P = 0.600); right box pair, average for four fish (rank sum test, P = 0.351). (D) Effects on frequency change during evoked chirping. Left box pair, same fish as in A and B (rank sum test, P = 0.977); right box pair, average of four fish (rank sum test, P = 0.889).
We conclude that of the three tuberous ELL segments, the CMS is necessary and apparently also sufficient for the processing of signals eliciting a JAR. Conversely, the LS appears to be necessary and sufficient to process external signals that evoke chirping. The behavioral role of the CLS is still unclear. It does not seem to be involved, however, in the encoding of signals yielding a JAR or evoking chirping.
DISCUSSION
Our findings are consistent with several earlier behavioral, physiological, and histochemical studies. For instance, pyramidal cells in each map respond differently to the frequency of sinusoidal amplitude modulations: most pyramidal cells in the CMS respond best to sinusoidal amplitude modulations of 1–3 Hz, whereas those in the LS prefer rates above 8 Hz (12). On the other hand, pyramidal cells in the CMS have a smaller receptive field size, thus showing higher spatial resolution than those in the LS (12–15). High spatial resolution is a prerequisite for the computational mechanisms controlling the JAR (9) and high temporal resolution is presumably required to encode the beat pattern that evokes chirps (15). Indeed, pyramidal cells in the LS encode simulations of brief chirps better than those in the CMS or CLS (15). Correspondingly, in behavioral experiments, the JAR is elicited most strongly by frequency differences of 1–6 Hz between the fish’s EOD (or its mimic) and the external signal, whereas frequency differences between 8 and 16 Hz evoke chirp responses best (9, 12, 22–24). Finally, serotonin, which seems to represent a neuromodulatory agent for the processing of conspecific communication signals in gymnotiform fish (14), is found at higher densities in LS than in CMS or CLS (13).
Numerous previous studies have demonstrated that the premotor pathways controlling communication behavior and the JAR are composed of separate pathways as well. They all converge at the level of the pacemaker nucleus, which controls the discharge rate of the electric organ. However, inputs modulating EOD rates in the context of communication behavior and jamming avoidance response, respectively, terminate on two different cell types and are mediated by different glutamate receptor subtypes (9, 28, 30–36). Hence, the segregated information flow in the context of two different behaviors appears to be conserved from the ELL all through the central nervous system to the pacemaker nucleus. But how are the intermediate brain structures able to sort out the incoming sensory information, extract higher order stimulus features, and activate the distinctly different premotor pathways that control the different behaviors? The projection patterns from the ELL to the next higher order structure of the ascending electrosensory pathway, the highly laminated torus semicircularis dorsalis, for instance, appear to differ only slightly between the various ELL segments (12). Yet the dorsal torus is known to contain neurons responding selectively only to signals occurring during the communication behavior and the JAR (9, 36). Refined anatomical tracing techniques might provide the tools needed to resolve differences in the connections from the various ELL maps to the 12 layers of the dorsal torus.
What is the reason for the distinctly distributed organization of the electrosensory system in these fish? Electrosensory systems might share a common evolutionary lineage with the mechanosensory (lateral line) system. It was suggested repeatedly that electrosensory brain structures could have evolved by duplication from mechanosensory areas (37, 38). Hence, it is tempting to speculate that duplication of existing brain maps could efficiently accommodate the increased information flow associated with a growth in the behavioral repertoire. This idea was originally proposed for the mirror image organization of sensory maps in mammalian cortex (39, 40). A further evolutionary increase in the complexity of sensory scenes and motor actions, such as in visually guided mammalian behavior, might eventually have required a greater flexibility in information processing. This might have yielded the shared use of circuit elements originally anchored in separate information streams by bridging between maps and, thus, resulted in the present distributed hierarchical organization of most vertebrate sensory systems. It is conceivable that the relatively simple nature of electric signals controlling a limited behavioral repertoire in electric fish caused the tuberous electrosensory system to retain this “primitive” character of a distinct modularity.
Acknowledgments
We thank T. Bullock, F. Gabbiani, C. Koch, S. Viete, and C. Wong for most helpful comments on earlier versions of the manuscript. Y. T. Yan, C. Rico, and D. Welsbie provided help with the histological tissue preparation. This work was supported by the University of California, Riverside, CA, and the National Institutes of Health.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CLS, centrolateral segment of the electrosensory lateral line lobe; CMS, centromedial segment of the electrosensory lateral line lobe; ELL, electrosensory lateral line lobe; EOD, electric organ discharge; JAR, jamming avoidance response; LS, lateral segment of the electrosensory lateral line lobe.
References
- 1.Shepherd G M, Pedersen P E, Greer C A. In: Perinatal Development. Krasnegor N A, Blass E M, Hofer M A, Smotherman W P, editors. New York: Academic; 1987. pp. 127–144. [Google Scholar]
- 2.Kaas J H, Garraghty P E. Curr Opin Neurobiol. 1991;1:248–251. doi: 10.1016/0959-4388(91)90085-l. [DOI] [PubMed] [Google Scholar]
- 3.Masterton R B. Trends Neurosci. 1992;15:280–285. doi: 10.1016/0166-2236(92)90077-l. [DOI] [PubMed] [Google Scholar]
- 4.Merigan W H, Maunsell J H R. Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- 5.Krauzlis R J, Basso M A, Wurtz R H. Science. 1997;276:1693–1696. doi: 10.1126/science.276.5319.1693. [DOI] [PubMed] [Google Scholar]
- 6.van Essen D C, Gallant J L. Neuron. 1994;13:1–10. doi: 10.1016/0896-6273(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 7.Konishi M. Trends Neurosci. 1988;11:163–168. [Google Scholar]
- 8.Viete S, Pena J L, Konishi M. J Neurosci. 1997;17:1815–1824. doi: 10.1523/JNEUROSCI.17-05-01815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiligenberg W. Neural Nets in Electric Fish. Cambridge, MA: MIT Press; 1991. [Google Scholar]
- 10.Carr C E, Maler L. In: Electroreception. Bullock T H, Heiligenberg W, editors. New York: Wiley; 1986. pp. 319–373. [Google Scholar]
- 11.Zakon, H. H. in Electroreception, eds. Bullock, T. H. & Heiligenberg, W. (Wiley, New York), pp. 103–156.
- 12.Shumway C A. J Neurosci. 1989;9:4388–4399. doi: 10.1523/JNEUROSCI.09-12-04388.1989. and 4400–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston S A, Maler L, Tinner B. J Chem Neuroanat. 1990;3:426–465. [PubMed] [Google Scholar]
- 14.Turner R W, Plant J R, Maler L. J Neurophysiol. 1996;76:2364–2382. doi: 10.1152/jn.1996.76.4.2364. [DOI] [PubMed] [Google Scholar]
- 15.Metzner W, Heiligenberg W. J Comp Physiol A. 1991;169:135–150. doi: 10.1007/BF00215861. [DOI] [PubMed] [Google Scholar]
- 16.Bastian J. In: Electroreception. Bullock T H, Heiligenberg W, editors. New York: Wiley; 1986. pp. 577–611. [Google Scholar]
- 17.Gabbiani F, Metzner W, Wessel R, Koch C. Nature (London) 1996;384:564–567. doi: 10.1038/384564a0. [DOI] [PubMed] [Google Scholar]
- 18.Maler L, Monaghan D. J Chem Neuroanat. 1991;4:39–61. doi: 10.1016/0891-0618(91)90031-7. [DOI] [PubMed] [Google Scholar]
- 19.Bottai, D., Dunn, R. J., Ellis, W. & Maler, L. (1997) J. Comp. Neurol., in press. [DOI] [PubMed]
- 20.Alves-Gomes J A, Orti G, Haygood M, Heiligenberg W, Meyer A. J Mol Biol Evol. 1995;12:298–318. doi: 10.1093/oxfordjournals.molbev.a040204. [DOI] [PubMed] [Google Scholar]
- 21.Bullock T H, Hamstra R H, Scheich H. J Comp Physiol A. 1972;77:1–48. [Google Scholar]
- 22.Heiligenberg W, Metzner W, Wong C J H, Keller C H. J Comp Physiol A. 1996;179:653–674. doi: 10.1007/BF00216130. [DOI] [PubMed] [Google Scholar]
- 23.Dye J. J Comp Physiol A. 1987;161:175–185. doi: 10.1007/BF00615239. [DOI] [PubMed] [Google Scholar]
- 24.Maler L, Ellis W. Behav Brain Res. 1987;25:75–81. doi: 10.1016/0166-4328(87)90046-5. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki M, Rose G, Heiligenberg W. Nature (London) 1988;336:173–176. doi: 10.1038/336173a0. [DOI] [PubMed] [Google Scholar]
- 26.Metzner W, Juranek J. J Neurosci Methods. 1997;76:143–150. doi: 10.1016/s0165-0270(97)00092-7. [DOI] [PubMed] [Google Scholar]
- 27.Olney J W. Adv Exp Med Biol. 1986;203:631–645. doi: 10.1007/978-1-4684-7971-3_48. [DOI] [PubMed] [Google Scholar]
- 28.Metzner W. J Neurosci. 1993;13:1862–1878. doi: 10.1523/JNEUROSCI.13-05-01862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maler L, Sas E, Johnston S, Ellis W. J Chem Neuroanat. 1991;4:1–38. doi: 10.1016/0891-0618(91)90030-g. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki M, Maler L, Rose G J, Heiligenberg W. J Comp Neurol. 1988;276:113–131. doi: 10.1002/cne.902760108. [DOI] [PubMed] [Google Scholar]
- 31.Dye J, Heiligenberg W, Keller C H, Kawasaki M. Proc Natl Acad Sci USA. 1989;86:8993–8997. doi: 10.1073/pnas.86.22.8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keller C H, Maler L, Heiligenberg W. J Comp Neurol. 1990;293:347–376. doi: 10.1002/cne.902930304. [DOI] [PubMed] [Google Scholar]
- 33.Spiro J E, Brose N, Heinemann S F, Heiligenberg W. J Neurosci. 1994;14:6289–6299. doi: 10.1523/JNEUROSCI.14-10-06289.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juranek J, Metzner W. J Comp Physiol A. 1997;181:393–414. [Google Scholar]
- 35.Juranek J, Metzner W. Soc Neurosci Abstr. 1997;27:101.14. [Google Scholar]
- 36.Metzner W, Viete S. Naturwissenschaften. 1996;83:6–14. and 71–77. [Google Scholar]
- 37.McCormick C A, Braford M R. In: Sensory Biology of Aquatic Animals. Atema J, Fay R R, Popper A N, Tavolga W N, editors. New York: Springer; 1988. pp. 733–756. [Google Scholar]
- 38.New J G, Singh S. Brain Behav Evol. 1994;34:43–50. doi: 10.1159/000113623. [DOI] [PubMed] [Google Scholar]
- 39.Allman J M, Kaas J H. Brain Res. 1971;31:85–105. doi: 10.1016/0006-8993(71)90635-4. [DOI] [PubMed] [Google Scholar]
- 40.Kaas J H. Contrib Sens Physiol. 1982;7:201–240. [Google Scholar]