Abstract
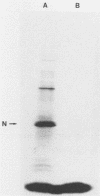
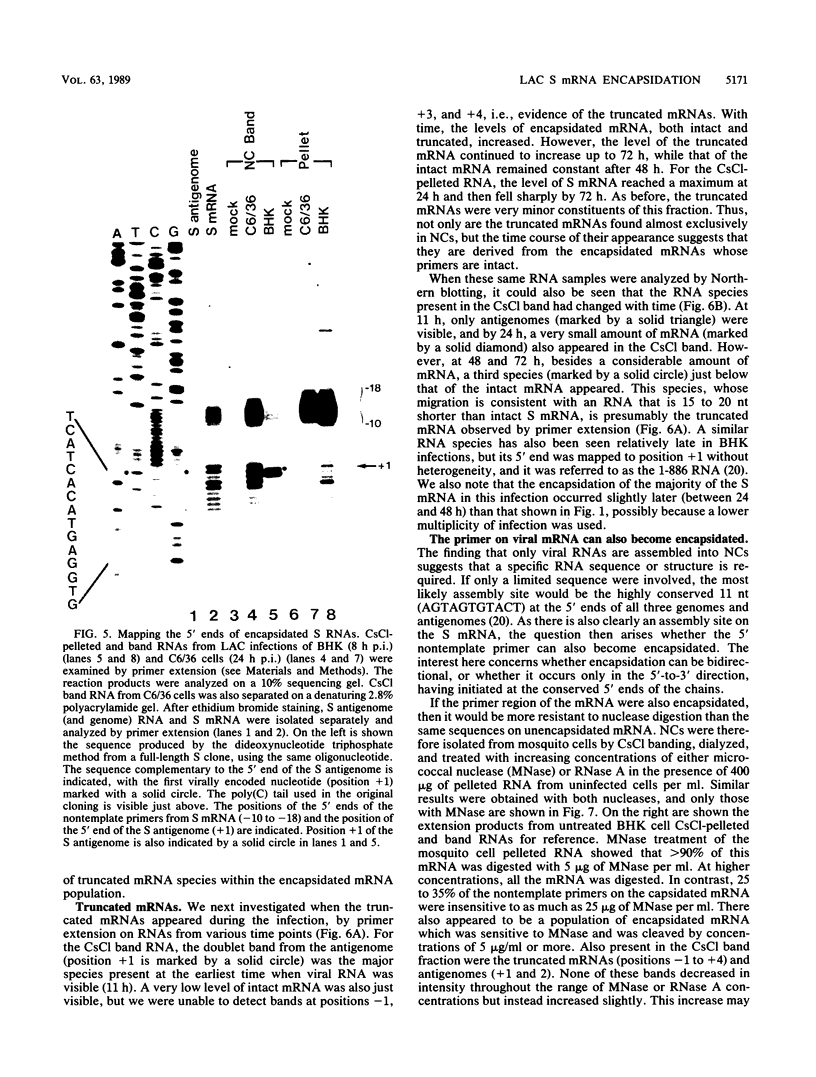
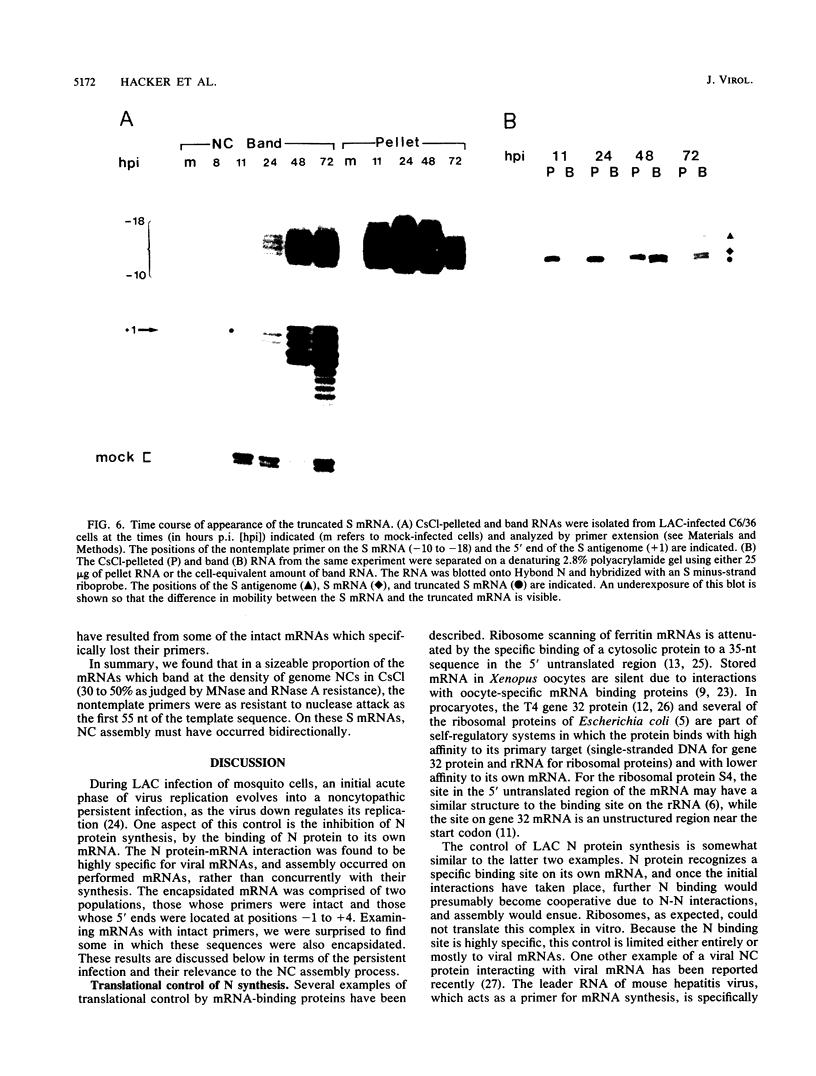
Within 24 to 48 h of La Crosse virus infection of mosquito cells, greater than 75% of the S mRNA was found to band in CsCl density gradients at the position of genome or antigenome nucleocapsids. The encapsidation of the S mRNA correlates with the repression of N protein synthesis in vivo, and the encapsidated S mRNA cannot be translated in vitro. Unlike genome and antigenome assembly, S mRNA assembly is a relatively slow process, which is not coupled to its synthesis. Within the encapsidated S mRNA population, three forms could be distinguished, those with intact primers which were or were not also assembled with N protein and those in which the primer and up to 3 template bases had been lost. We suggest that genome replication, but not transcription, is down regulated with time in mosquito cells for reasons that are unclear. The pool of unassembled N protein then increased to the point at which it began to interact with its own mRNA, as this mRNA also contains what is considered to be the assembly site, i.e., the conserved sequences at the 5' ends of all genome and antigenome chains. This lead to the assembly of the entire mRNA, except for the nontemplate primer. Some of the primers were then also assembled with N protein, whereas others were digested to produce truncated mRNAs.
Full text
PDF

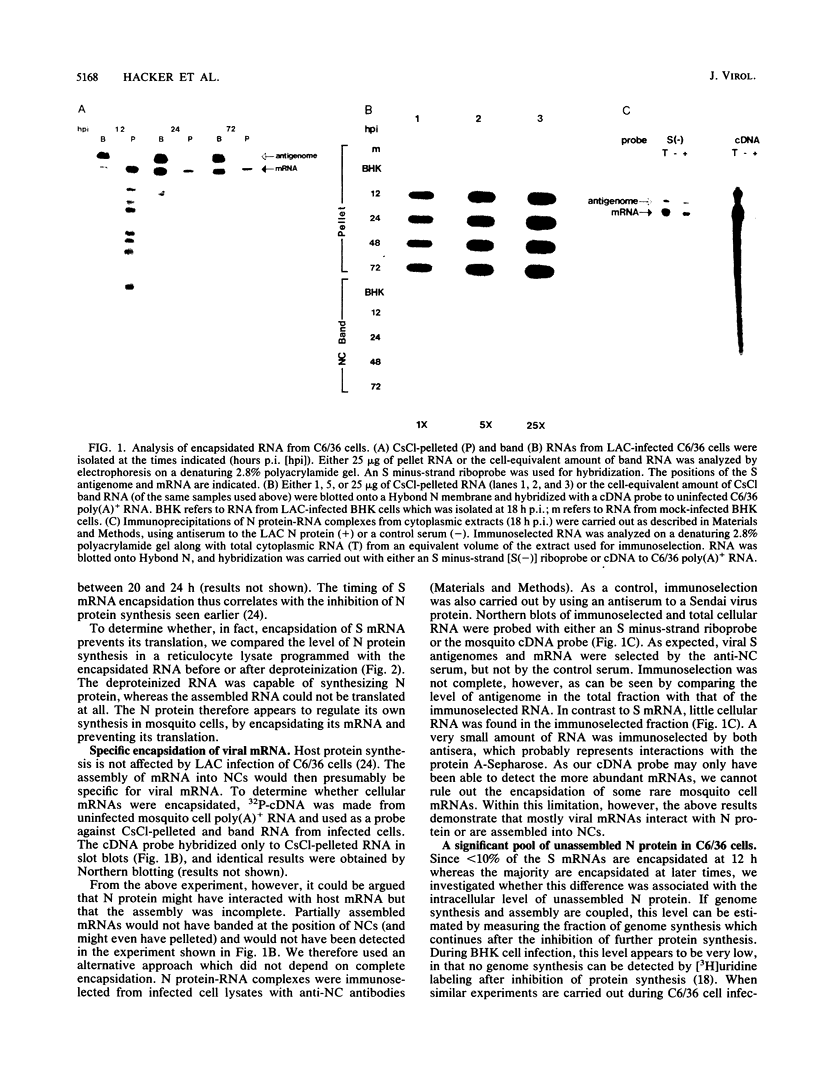
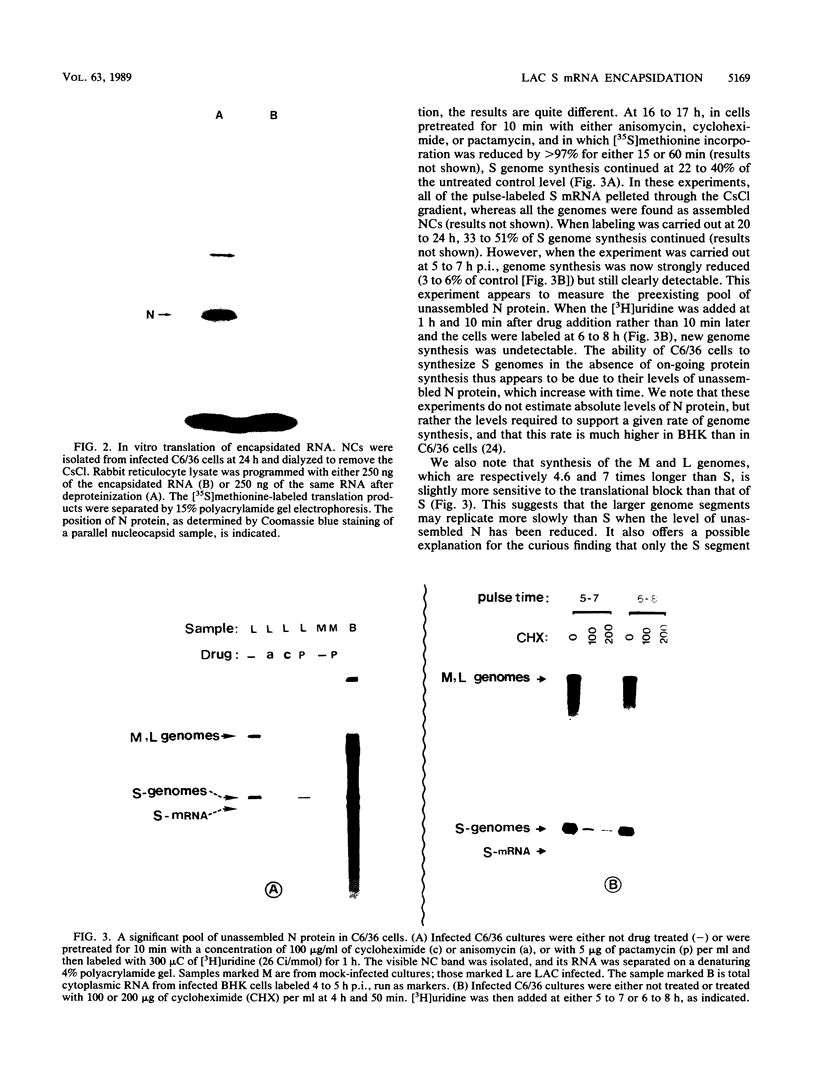
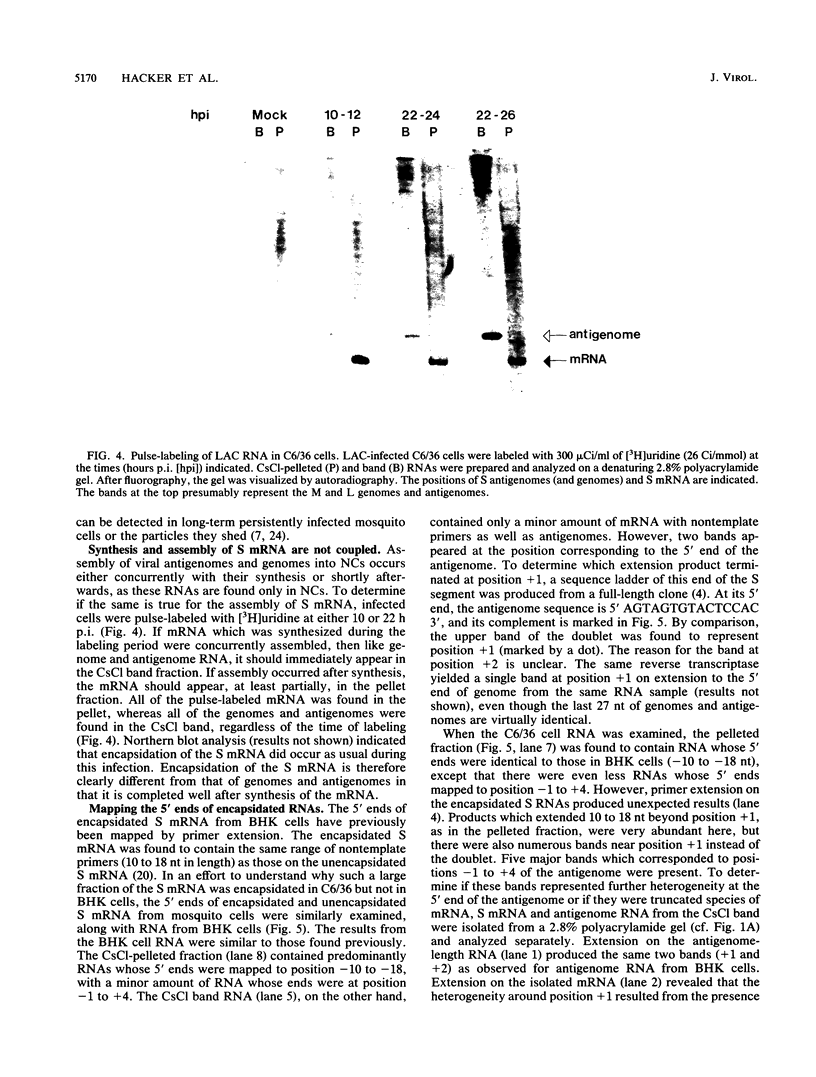


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Gay M. E., Matsuoko Y. Nonviral heterogeneous sequences are present at the 5' ends of one species of snowshoe hare bunyavirus S complementary RNA. Nucleic Acids Res. 1983 Sep 24;11(18):6409–6418. doi: 10.1093/nar/11.18.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Cabradilla C. D., Jr, Holloway B. P., Obijeski J. F. Molecular cloning and sequencing of the La Crosse virus S RNA. Virology. 1983 Jul 30;128(2):463–468. doi: 10.1016/0042-6822(83)90271-4. [DOI] [PubMed] [Google Scholar]
- Dean D., Nomura M. Feedback regulation of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3590–3594. doi: 10.1073/pnas.77.6.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckman I. C., Draper D. E. S4-alpha mRNA translation regulation complex. II. Secondary structures of the RNA regulatory site in the presence and absence of S4. J Mol Biol. 1987 Jul 20;196(2):323–332. doi: 10.1016/0022-2836(87)90693-0. [DOI] [PubMed] [Google Scholar]
- Elliott R. M., Wilkie M. L. Persistent infection of Aedes albopictus C6/36 cells by Bunyamwera virus. Virology. 1986 Apr 15;150(1):21–32. doi: 10.1016/0042-6822(86)90262-x. [DOI] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh's Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978 Sep;40(3):531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Kick D., Barrett P., Cummings A., Sommerville J. Phosphorylation of a 60 kDa polypeptide from Xenopus oocytes blocks messenger RNA translation. Nucleic Acids Res. 1987 May 26;15(10):4099–4109. doi: 10.1093/nar/15.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch H. M., Allet B. Nucleotide sequences involved in bacteriophage T4 gene 32 translational self-regulation. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4937–4941. doi: 10.1073/pnas.79.16.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisch H. M., Bolle A., Epstein R. H. Regulation of the synthesis of bacteriophage T4 gene 32 protein. J Mol Biol. 1974 Sep 5;88(1):89–104. doi: 10.1016/0022-2836(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. E., Short N. J., Dalgarno L. Bunyamwera virus replication in cultured Aedes albopictus (mosquito) cells: establishment of a persistent viral infection. J Virol. 1981 Jun;38(3):1015–1024. doi: 10.1128/jvi.38.3.1015-1024.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Murphy F. A. Bunyaviridae: recent biochemical developments. J Gen Virol. 1977 Oct;37(1):1–14. doi: 10.1099/0022-1317-37-1-1. [DOI] [PubMed] [Google Scholar]
- Patterson J. L., Holloway B., Kolakofsky D. La Crosse virions contain a primer-stimulated RNA polymerase and a methylated cap-dependent endonuclease. J Virol. 1984 Oct;52(1):215–222. doi: 10.1128/jvi.52.1.215-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L., Kolakofsky D. Characterization of La Crosse virus small-genome transcripts. J Virol. 1984 Mar;49(3):680–685. doi: 10.1128/jvi.49.3.680-685.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield J. S., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Holmes I. H., Horzinek M. C., Mussgay M., Oker-Blom N., Russell P. K. Bunyaviruses and Bunyaviridae. Intervirology. 1975;6(1):13–24. doi: 10.1159/000149449. [DOI] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. La Crosse virus infection of mammalian cells induces mRNA instability. J Virol. 1988 Jan;62(1):27–32. doi: 10.1128/jvi.62.1.27-32.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Translational requirement of La Crosse virus S-mRNA synthesis: in vivo studies. J Virol. 1987 Jan;61(1):96–103. doi: 10.1128/jvi.61.1.96-103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Kolakofsky D. Unusual transcripts in La Crosse virus-infected cells and the site for nucleocapsid assembly. J Virol. 1987 Mar;61(3):667–672. doi: 10.1128/jvi.61.3.667-672.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D., Smith L. D. Reversible inhibition of translation by Xenopus oocyte-specific proteins. Nature. 1984 May 24;309(5966):378–380. doi: 10.1038/309378a0. [DOI] [PubMed] [Google Scholar]
- Rossier C., Raju R., Kolakofsky D. LaCrosse virus gene expression in mammalian and mosquito cells. Virology. 1988 Aug;165(2):539–548. doi: 10.1016/0042-6822(88)90598-3. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Russel M., Gold L., Morrissett H., O'Farrell P. Z. Translational, autogenous regulation of gene 32 expression during bacteriophage T4 infection. J Biol Chem. 1976 Nov 25;251(22):7263–7270. [PubMed] [Google Scholar]
- Stohlman S. A., Baric R. S., Nelson G. N., Soe L. H., Welter L. M., Deans R. J. Specific interaction between coronavirus leader RNA and nucleocapsid protein. J Virol. 1988 Nov;62(11):4288–4295. doi: 10.1128/jvi.62.11.4288-4295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]