Abstract
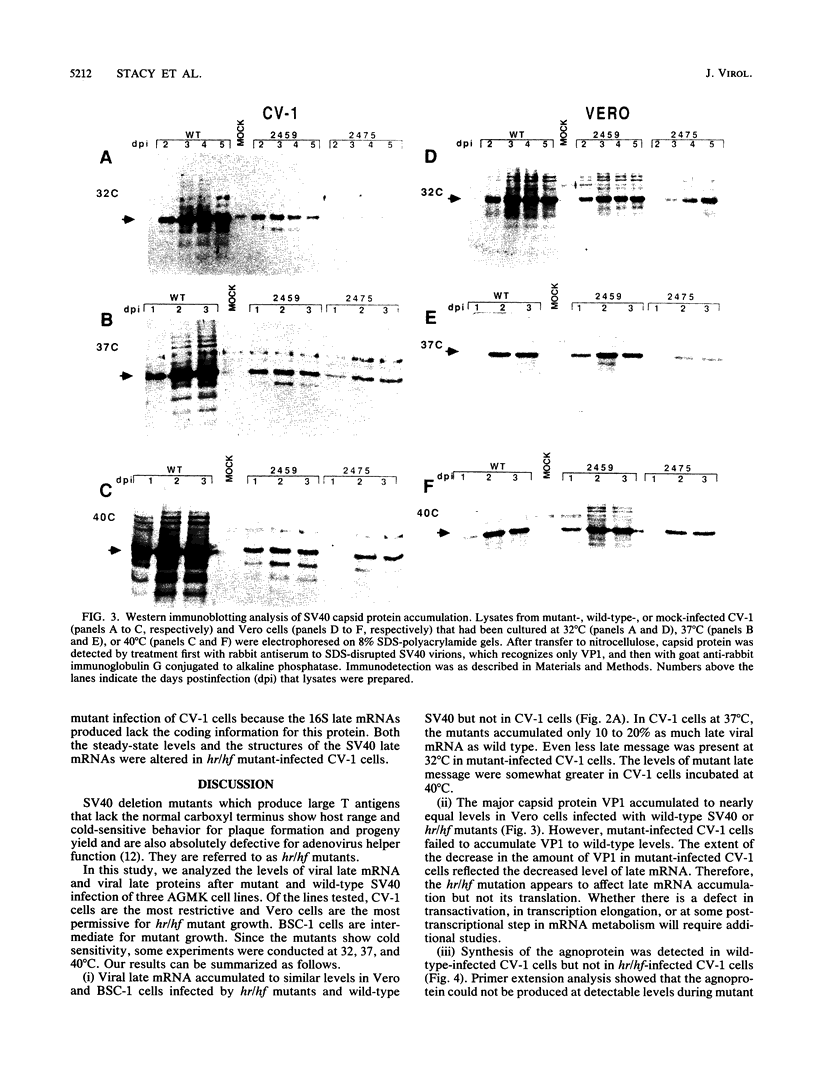
Simian virus 40 (SV40) deletion mutants dlA2459 and dlA2475 express T antigens that lack the normal carboxy terminus. These mutants are called host range/helper function (hr/hf) mutants because they form plaques at 37 degrees C on BSC-1 and Vero monkey kidney cell lines but not on CV-1p monkey kidney cells. Wild-type SV40 can provide a helper function to permit growth of human adenoviruses in monkey kidney cells; the hr/hf mutants cannot. Progeny yields of hr/hf mutants are also cold sensitive in all cell lines tested. Patterns of viral macromolecular synthesis in three cell lines (Vero, BSC-1, and CV-1) at three temperatures (40, 37, and 32 degrees C) were examined to determine the nature of the growth defect of hr/hf mutants. Mutant viral DNA replication was similar to that of the wild type in all three cell lines, indicating that the mutations affect late events in the viral lytic cycle. In mutant-infected Vero cells, in which viral yields were highest, late mRNA levels were similar to those observed during wild-type infection. Levels of viral late mRNA from mutant-infected CV-1 and BSC-1 cells at 32 and 37 degrees C were reduced relative to those of wild-type-infected cells. The steady-state level of the major viral capsid protein, VP1, in mutant-infected CV-1 cells was reduced to the same extent as was late mRNA. The synthesis of agnoprotein could not be detected in mutant-infected CV-1 cells but was readily detected in CV-1 cells infected by wild-type SV40. Primer extension analyses indicated that most late mRNAs from mutant-infected CV-1 cells utilize start sites downstream from the major wild-type cap site (nucleotide 325) and the agnoprotein initiation codon (nucleotide 335). These results indicate that deletion of the carboxyl-terminal domain of T antigen affects viral late mRNA production, both quantitatively and qualitatively. The agnoprotein is detected late in the wild-type SV40 lytic cycle and is thought to play a role in the assembly or maturation of virions. Reduced hr/hf progeny yields could result from decreased capsid protein synthesis and, in the absence of detectable levels of agnoprotein, from inefficient use of available capsid proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Posttranscriptional block to synthesis of a human adenovirus capsid protein in abortively infected monkey cells. J Mol Appl Genet. 1983;2(1):31–43. [PubMed] [Google Scholar]
- Barkan A., Welch R. C., Mertz J. E. Missense mutations in the VP1 gene of simian virus 40 that compensate for defects caused by deletions in the viral agnogene. J Virol. 1987 Oct;61(10):3190–3198. doi: 10.1128/jvi.61.10.3190-3198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradley M. K., Hudson J., Villanueva M. S., Livingston D. M. Specific in vitro adenylylation of the simian virus 40 large tumor antigen. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6574–6578. doi: 10.1073/pnas.81.21.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Bolen J. B., Radonovich M., Salzman N., Khoury G. Stimulation of simian virus 40 late gene expression by simian virus 40 tumor antigen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2040–2044. doi: 10.1073/pnas.81.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Khoury G. trans Activation of the simian virus 40 late transcription unit by T-antigen. Mol Cell Biol. 1985 Jun;5(6):1391–1399. doi: 10.1128/mcb.5.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell S., Alwine J. C. Simian virus 40 agnoprotein facilitates perinuclear-nuclear localization of VP1, the major capsid protein. J Virol. 1986 Dec;60(3):1055–1061. doi: 10.1128/jvi.60.3.1055-1061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Crawford L. V., Berg P. Simian virus 40 mutants with deletions at the 3' end of the early region are defective in adenovirus helper function. J Virol. 1979 Jun;30(3):683–691. doi: 10.1128/jvi.30.3.683-691.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Stacy T. P. Biological properties of simian virus 40 host range mutants lacking the COOH-terminus of large T antigen. Virology. 1987 Nov;161(1):170–180. doi: 10.1016/0042-6822(87)90183-8. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Tornow J., Clark R., Tjian R. Properties of the simian virus 40 (SV40) large T antigens encoded by SV40 mutants with deletions in gene A. J Virol. 1986 Feb;57(2):539–546. doi: 10.1128/jvi.57.2.539-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Eron L. Post-transcriptional restriction of human adenovirus expression in monkey cells. J Virol. 1975 May;15(5):1256–1261. doi: 10.1128/jvi.15.5.1256-1261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G., Lewis J. B., Grodzicker T., Bothwell A. Characterization of a fused protein specified by the adenovirus type 2-simian virus 40 hybrid Ad2+ND1 dp2. J Virol. 1979 Apr;30(1):201–217. doi: 10.1128/jvi.30.1.201-217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Goldman N., Brown M., Khoury G. Modification of SV40 T antigen by poly ADP-ribosylation. Cell. 1981 May;24(2):567–572. doi: 10.1016/0092-8674(81)90347-0. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Lewis J. B., Anderson C. W. Conditional lethal mutants of adenovirus type 2-simian virus 40 hybrids. II. Ad2+ND1 host-range mutants that synthesize fragments of the Ad2+ND1 30K protein. J Virol. 1976 Aug;19(2):559–571. doi: 10.1128/jvi.19.2.559-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman G., Fiers W. Characterization of the 5'-terminal capped structures of late simian virus 40-specific mRNA. J Virol. 1978 Mar;25(3):824–830. doi: 10.1128/jvi.25.3.824-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis D. L., Butel J. S. Modification of simian virus 40 large tumor antigen by glycosylation. Virology. 1985 Mar;141(2):173–189. doi: 10.1016/0042-6822(85)90250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Jessel D., Hudson J., Landau T., Tenen D., Livingston D. M. Interaction of partially purified simian virus 40 T antigen with circular viral DNA molecules. Proc Natl Acad Sci U S A. 1975 May;72(5):1960–1964. doi: 10.1073/pnas.72.5.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Alwine J. C. Activation of the SV40 late promoter: direct effects of T antigen in the absence of viral DNA replication. Cell. 1984 Feb;36(2):381–389. doi: 10.1016/0092-8674(84)90231-9. [DOI] [PubMed] [Google Scholar]
- Khalili K., Brady J., Pipas J. M., Spence S. L., Sadofsky M., Khoury G. Carboxyl-terminal mutants of the large tumor antigen of simian virus 40: a role for the early protein late in the lytic cycle. Proc Natl Acad Sci U S A. 1988 Jan;85(2):354–358. doi: 10.1073/pnas.85.2.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Anderson C. W. Block to multiplication of adenovirus serotype 2 in monkey cells. J Virol. 1975 Dec;16(6):1650–1668. doi: 10.1128/jvi.16.6.1650-1668.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Klockmann U., Deppert W. Acylated simian virus 40 large T-antigen: a new subclass associated with a detergent-resistant lamina of the plasma membrane. EMBO J. 1983;2(7):1151–1157. doi: 10.1002/j.1460-2075.1983.tb01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manos M. M., Gluzman Y. Genetic and biochemical analysis of transformation-competent, replication-defective simian virus 40 large T antigen mutants. J Virol. 1985 Jan;53(1):120–127. doi: 10.1128/jvi.53.1.120-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M. Mutations near the carboxyl terminus of the simian virus 40 large tumor antigen alter viral host range. J Virol. 1985 May;54(2):569–575. doi: 10.1128/jvi.54.2.569-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polvino-Bodnar M., Cole C. N. Construction and characterization of viable deletion mutants of simian virus 40 lacking sequences near the 3' end of the early region. J Virol. 1982 Aug;43(2):489–502. doi: 10.1128/jvi.43.2.489-502.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C., Beck Y., Gidoni D., Oren M., Shure H. DNA binding and sedimentation properties of SV40 T antigens synthesized in vivo and in vitro. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):123–130. doi: 10.1101/sqb.1980.044.01.014. [DOI] [PubMed] [Google Scholar]
- RABSON A. S., O'CONOR G. T., BEREZESKY I. K., PAUL F. J. ENHANCEMENT OF ADENOVIRUS GROWTH IN AFRICAN GREEN MONKEY KIDNEY CELL CULTURES BY SV40. Proc Soc Exp Biol Med. 1964 May;116:187–190. doi: 10.3181/00379727-116-29197. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Weissman S. M. Gaps and duplicated sequences in the leaders of SV40 16S RNA. Nucleic Acids Res. 1978 Nov;5(11):4195–4213. doi: 10.1093/nar/5.11.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick J., Shenk T. Simian virus 40 agnoprotein facilitates normal nuclear location of the major capsid polypeptide and cell-to-cell spread of virus. J Virol. 1986 Dec;60(3):1098–1106. doi: 10.1128/jvi.60.3.1098-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F. Isolation and analysis of adenovirus type 5 mutants containing deletions in the gene encoding the DNA-binding protein. J Virol. 1985 Dec;56(3):767–778. doi: 10.1128/jvi.56.3.767-778.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Knippers R. DNA helicase activity of SV40 large tumor antigen. EMBO J. 1986 Aug;5(8):1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Rundell K., Collins J. K. Modification of simian virus 40 protein A. J Virol. 1977 Feb;21(2):647–657. doi: 10.1128/jvi.21.2.647-657.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Tornow J., Cole C. N. Intracistronic complementation in the simian virus 40 A gene. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6312–6316. doi: 10.1073/pnas.80.20.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornow J., Cole C. N. Nonviable mutants of simian virus 40 with deletions near the 3' end of gene A define a function for large T antigen required after onset of viral DNA replication. J Virol. 1983 Sep;47(3):487–494. doi: 10.1128/jvi.47.3.487-494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornow J., Polvino-Bodnar M., Santangelo G., Cole C. N. Two separable functional domains of simian virus 40 large T antigen: carboxyl-terminal region of simian virus 40 large T antigen is required for efficient capsid protein synthesis. J Virol. 1985 Feb;53(2):415–424. doi: 10.1128/jvi.53.2.415-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. T., Berg P., Villarreal L. P. Simian virus 40-rabbit beta-globin recombinants lacking late mRNA splice sites express cytoplasmic RNAs with altered structures. J Virol. 1982 Apr;42(1):262–274. doi: 10.1128/jvi.42.1.262-274.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]