Abstract
During embryogenesis, pluripotent stem cells segregate into daughter lineages of progressively restricted developmental potential. In vitro, this process has been mimicked by the controlled differentiation of embryonic stem cells into neural precursors. To explore the developmental potential of these cell-culture-derived precursors in vivo, we have implanted them into the ventricles of embryonic rats. The transplanted cells formed intraventricular neuroepithelial structures and migrated in large numbers into the brain tissue. Embryonic-stem-cell-derived neurons, astrocytes, and oligodendrocytes incorporated into telencephalic, diencephalic, and mesencephalic regions and assumed phenotypes indistinguishable from neighboring host cells. These observations indicate that entirely in vitro-generated neural precursors are able to respond to environmental signals guiding cell migration and differentiation and have the potential to reconstitute neuronal and glial lineages in the central nervous system.
The ability to isolate, proliferate, and genetically manipulate embryonic stem (ES) cells is one of the major achievements in experimental biology (1, 2). The totipotency of these cells has kindled numerous efforts to generate tissue-specific precursors from ES cells in vitro. The controlled differentiation of ES cells into stem cells of defined lineages provides experimental access to early embryonic development and may eventually lead to alternative donor sources for tissue reconstruction. A central question concerning the biology of ES-cell-derived precursors is to what extent these cells resemble their in vivo counterparts. Are precursor cells generated outside the context of a multicellular organism sufficiently responsive to positional cues to participate in the development and histogenesis of a living host? Hematopoietic stem cells derived from ES cells have, indeed, been shown to reconstitute the lymphoid, myeloid, and erythroid lineages after transplantation into irradiated mice (3). During the last 3 years, several groups have demonstrated that mature neurons and glia can be derived from ES cells in vitro (4–7). Recently, we have reported an efficient procedure for the generation of proliferative neural precursors from ES cells. In vitro, these precursors generate functional neurons, astrocytes, and oligodendrocytes (8). In vivo reconstitution would be a more stringent test of the potential of ES cells to acquire central nervous system fates. The limited self-renewal in the adult mammalian brain precludes the kind of ablation and reconstitution experiments used to study in vivo differentiation of hematopoietic progenitors. We have, therefore, used a different approach and introduced ES-cell-derived neural precursors into the developing brain. Previous studies have revealed that primary neuroepithelial precursors implanted into the ventricle of embryonic rats incorporate extensively into the host brain and undergo region-specific differentiation into neurons and glia (9–11). Herein, we show that ES-cell-derived neural precursors grafted into the embryonic ventricle migrate into the host brain and contribute to all three principal lineages of the nervous system. Our results suggest that neuroepithelial precursors derived from ES cells in the absence of positional cues can migrate and differentiate according to local signals in the host environment.
MATERIALS AND METHODS
ES Cell Culture.
ES cells (line J1; ref. 12) were maintained on γ-irradiated fibroblasts in DMEM containing 20% fetal calf serum, 0.1 mM 2-mercaptoethanol, nucleosides, nonessential amino acids, and human recombinant leukemia inhibitory factor (1000 units/ml). Cells were passaged once onto gelatin-coated dishes and then aggregated to form embryoid bodies in bacterial dishes in the absence of leukemia inhibitory factor. Four-day-old embryoid bodies were plated in tissue culture dishes and propagated for 5–12 days in ITSFn medium (DMEM/F-12 containing insulin at 5 μg/ml, transferrin at 50 μg/ml, 30 nM selenium chloride, and fibronectin at 5 μg/ml; ref. 8).
Intrauterine Transplantation.
Cells were trypsinized and triturated to single-cell suspensions in the presence of 0.1% DNase. Timed-pregnant Sprague–Dawley rats were anesthetized with ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg), and 0.1–1 × 106 cells were injected into the telencephalic vesicle of each embryo as described (11).
Immunohistochemistry.
Zero to 15 days after spontaneous birth, recipients were anesthetized and perfused with 4% paraformaldhyde in PBS (stillborn recipients were fixed by immersion). Serial 50-μm Vibratome sections were characterized with antibodies to microtubule-associated protein 2 (Sigma, dilution, 1:200), nestin (dilution, 1:1,000), glial fibrillary acidic protein (GFAP; ICN, dilution, 1:100), (CNPase; Sigma, dilution, 1:200), neurofilament (SMI311, Sternberger Monoclonals, Baltimore, MD; dilution, 1:500), NeuN (Chemicon, dilution, 1:50), tyrosine hydroxylase (Eugene Tech Intl., Ridgefield Park, NJ; dilution, 1:200), M2 and M6 (refs. 13 and 14; dilution, 1:10). Antigens were visualized by using appropriate fluorophore- or peroxidase-conjugated secondary antibodies. To assay alkaline phosphatase activity, sections were incubated at 37°C in 100 mM Tris⋅HCl, pH 9.5/100 mM NaCl/50 mM MgCl2/nitroblue tetrazolium at 0.3 mg/ml/5-bromo-4-chloro-3-indolyl phosphate at 0.175 mg/ml. After 3–10 min, the staining was stopped by transferring the sections to 10 mM Tris⋅HCl/1 mM EDTA. Specimens were examined on Zeiss Axioplan, Axiovert, and Laser Scan microscopes.
In Situ Hybridization.
Donor cells were identified by using a digoxigenin-end-labeled oligonucleotide probe to the mouse major satellite (15). DNA–DNA in situ hybridization was performed as described (11). Briefly, sections were treated with Pronase at 25 μg/ml in 2× SSC/5 mM EDTA for 15 min at 37°C, dehydrated, and denatured in 70% formamide/2× SSC at 85°C for 12 min. After dehydration in ice-cold ethanols, sections were hybridized overnight at 37°C in 65% formamide/2× SSC/salmon sperm DNA at 250 μg/ml. Washes were 50% formamide/2× SSC (30 min, 37°C) and 0.5× SSC (37°C, 15 min). Hybridized probe was detected by using alkaline phosphatase or fluorophore-conjugated antibodies to digoxigenin (Boehringer).
RESULTS
Widespread Incorporation of ES-Cell-Derived Neural Precursors into the Developing Brain.
For transplantation, 4-day embryoid bodies were plated on tissue culture dishes and grown in ITSFn medium. This medium has previously been shown to strongly select for neural precursors (8). During the first 72 h in ITSFn, a large proportion of the cells died. Most of the remaining cells acquired an elongated phenotype strongly reminiscent of neuroepithelial precursor cells. These cells also expressed nestin, an intermediate filament typically present in neural precursor cells (16). After 6 days in ITSFn medium, typically more than 80% of the cells were nestin-positive. The remaining cells showed varied morphologies with focal expression of SSEA-1 and keratin 8, i.e., antigens typically expressed in undifferentiated embryonic tissues and primitive ectoderm (refs. 17 and 18; data not shown). After 5–12 days in ITSFn, cells were harvested and used for transplantation. Recipient animals were grafted between embryonic day (E) 16 and E18 and sacrificed between postnatal day (P) 0 and P15. Clusters of donor cells were detected in the ventricles of all successfully injected pups (see below). In situ hybridization revealed that large numbers of mouse cells left the ventricle and migrated into various host brain regions, including cortex, striatum, septum, thalamus, hypothalamus, and tectum (Figs. 1 and 2 A, E, F, and K and Table 1). The transplanted cells integrated individually into the host tissue and were only detectable by virtue of their genetic difference. The number of incorporated cells varied considerably among individual recipients and brain regions. Quantitative stereology and a detailed assessment of donor cell survival and proliferation will be required to assess what proportion of the transplanted cells integrates into the host brain. Preliminary cell counts have revealed up to 650 incorporated cells in single coronal 50-μm sections.
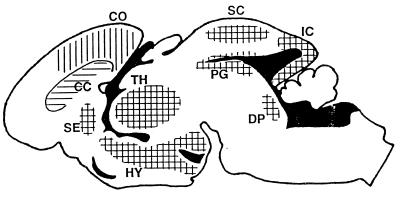
Figure 1.
Distribution of ES-cell-derived neural precursors after implantation into the telencephalic vesicle of E16–E18 rats. The schematic represents a midsagittal section through the brain of a newborn recipient. After leaving the ventricular system (solid areas), neurons (vertical lines) and astrocytes (horizontal lines) occupy overlying territories. Donor-derived neurons integrate preferentially into gray matter regions exhibiting neurogenesis until or beyond the time of implantation. ES-cell-derived astrocytes also incorporate into white matter regions such as the corpus callosum (CC). CO, cortex; DP, dorsal pontine area; HY, hypothalamus; IC, inferior colliculus; PG, periaqueductal gray; SC, superior colliculus; SE, septum; TH, thalamus. Donor-derived neurons and astrocytes were also detected in hippocampus (Fig. 3B), olfactory bulb (Fig. 4 A and B), and striatum (Fig. 2E).
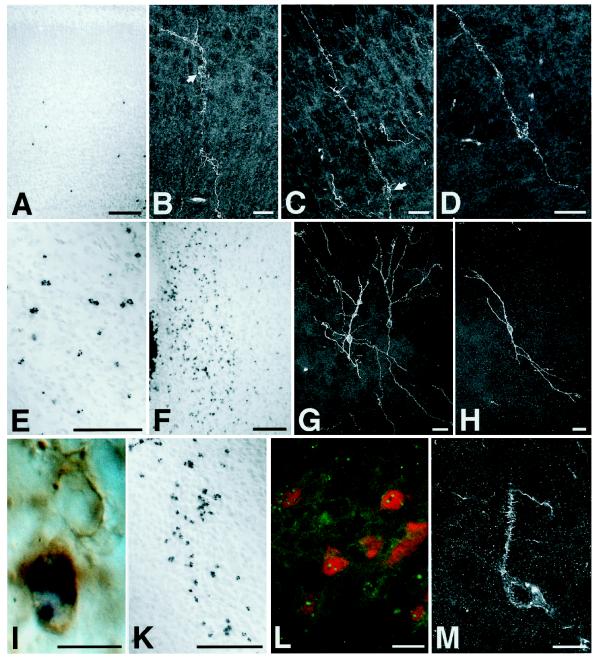
Figure 2.
ES-cell-derived neural precursors injected into the telencephalic vesicle of fetal rats incorporate individually into a variety of host brain regions and differentiate into neurons. Donor cells are identified by in situ hybridization using a digoxigenin-labeled probe to mouse satellite DNA (A, E, F, and I–L). Immunofluorescence detection of the mouse-specific antigen M6 and confocal laser microscopy were used to reconstruct individual neuronal profiles (B–D, G, H, and M). (A–D) Six days after injection into the telencephalic vesicle of an E17 rat, donor cells have left the ventricle and incorporated into the host cortex. The ES-cell-derived neurons show prominent apical dendrites and basal axons entering the corpus callosum, a morphology appropriate for cortical projection neurons (arrows: perikaryon). Note the characteristic pyramidal morphology in D. (E) Incorporated donor cells in the striatum of a 2-week-old rat transplanted at E18. (F–H) Donor-derived cells in the host hypothalamus. In contrast to cortex, neurons incorporating into the diencephalon frequently exhibited multipolar morphologies (G and H). (I) Host- and donor-derived neurons in the septum of a newborn rat. Both neurons show expression of microtubule-associated protein 2; the donor-derived cell is identified by in situ hybridization. (K–M) Incorporated cells in the host thalamus of newborn (K) and 2-week-old rats (L and M). In L, ES-cell-derived neurons are visualized by fluorescence in situ hybridization (green dots) and subsequent immunofluorescence analysis with an antibody to the nuclear neuronal antigen NeuN (red). Note the mature neuronal phenotype of the integrated cells with the presence of dendritic spines (M). [Bars = 100 μm (A, E, F, and K), 20 μm (B–D, G, H, L, and M), and 10 μm (I).]
Table 1.
Differentiation of ES-cell-derived neural precursors after transplantation into the embryonic rat brain
| Age at analysis | Parenchyma
|
Ventricle
|
|||
|---|---|---|---|---|---|
| Neurons | Glia | NEF | AP | NNT | |
| P0 | TDM | D | + | + | − |
| P0 | TDM | T | − | + | − |
| P0 | TD | − | + | − | − |
| P0 | TM | − | − | + | − |
| P0 | TDM | T | − | ++ | − |
| P0 | D | D | + | + | − |
| P1 | TDM | − | − | ++ | − |
| P1 | TD | − | − | ++ | − |
| P1 | TDM | − | − | + | − |
| P1 | TD | − | − | + | − |
| P1 | TDM | TDM | − | + | − |
| P1 | TDM | − | − | + | − |
| P1 | TDM | D | − | + | − |
| P1 | TDM | TM | − | ++ | − |
| P15 | DM | DM | + | + | + |
| P15 | TDM | TD | − | − | + |
| P15 | TDM | TDM | + | − | + |
| P15 | TM | TM | + | + | + |
| P15 | T | TD | + | − | + |
| P15 | TDM | TM | + | − | + |
| P15 | TD | TD | + | + | + |
ES cells (line J1) aggregated to embryoid bodies and grown for 5–12 days in ITSFn medium were injected into the ventricle of E16–E18 rats. Recipients were sacrificed between P0 and P15 and donor-derived neurons were identified by DNA in situ hybridization in conjunction with immunohistochemical detection of NeuN or by expression of M6 and unequivocal morphological criteria (presence of dendrites and axons). ES-cell-derived astrocytes were detected with an antibody to M2 or by DNA in situ hybridization in conjunction with immunohistochemical detection of GFAP. Intraventricular donor cell clusters were assayed for the presence of nestin-positive neuroepithelial formations (NEF), clusters of undifferentiated, alkaline phosphatase-positive cells (AP; ++, numerous clusters; +, occasional clusters), and differentiated nonneural tissue (NNT). Each row represents one recipient animal. The integration patterns show considerable interindividual variability. There is an increase of glial cells and a decrease of AP-positive cells with increasing survival time. T, telencephalon; D, diencephalon; M, mesencephalon.
Differentiation into Neurons and Glia.
The differentiation of the incorporated cells was assessed by using antibodies to cell-type-specific antigens in conjunction with a mouse-specific DNA probe or mouse-specific antibodies to M6 and M2 (13, 14). Hybridized neurons expressing the neuronal antigens NeuN (19) and microtubule-associated protein 2 were detected at tel-, di-, and mesencephalic levels (Fig. 2 I and L). The shape, size, and orientation of these cells were indistinguishable from adjacent host neurons (Fig. 2I). Confocal laser microscopy allowed detailed reconstruction of individual phenotypes. ES-cell-derived neurons exhibited characteristic polar morphologies with segregation of neurites into dendrites and axons (Fig. 2 B–D and G). Both classes of neurites frequently extended several hundred micrometers into the adjacent host neuropil (Fig. 2 G and H). Donor-derived neurons were readily detectable at birth but appeared to undergo further morphological maturation in the postnatal period. The example in Fig. 2M shows an ES-cell-derived neuron with prominent dendritic spines in the thalamus of a 2-week-old host. Neurons integrating into the host cortex frequently displayed morphologies of projection neurons with long apical dendrites reaching into the superficial cortical layers and basal axons extending several hundred micrometers into the corpus callosum (Fig. 2 B–D). An example of a ES-cell-derived pyramidal neuron in the cortex of a neonatal host is shown in Fig. 2D. The donor neurons generated an extensive axonal network throughout the host gray and white matter, reaching from the most rostral regions such as the olfactory bulb to the brainstem (Fig. 3). Within the white matter, donor-derived axons frequently assembled into prominent fiber bundles running alongside host axons through the major axonal trajectories, including the corpus callosum (Fig. 3A), anterior commissure, striatal fiber bundles (Fig. 3C), and various other endogenous fiber tracts. Gray matter regions exhibiting dense donor-derived axonal networks included cortex (Fig. 3A), hippocampus (Fig. 3B), septum, striatum, thalamus (Fig. 3D), hypothalamus, tegmentum, tectum, and brainstem.
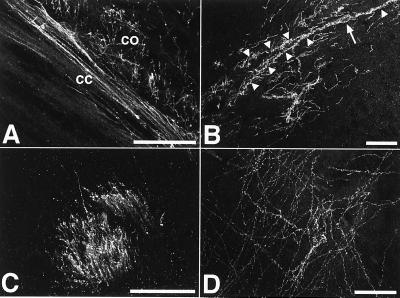
Figure 3.
Extensive axonal innervation of the host brain. The ES-cell-derived neurons generated a dense axonal network within the recipient brains. Abundant M6-positive axons were found at all levels in both gray and white matter. (A) Donor-derived axons in corpus callosum (cc) and deep layer cortex (co) of a 2-week-old recipient. (B) Axonal innervation of the hippocampal stratum oriens. The M6 immunofluorescence also depicts the perikaryon (arrow) and dendrites (arrowheads) of a large horizontal neuron in the upper stratum oriens. The morphology of this cell is very similar to the outline of Golgi-impregnated horizontal neurons in this area (20). (C) Abundant donor-derived axons in a striatal fiber tract of 2-week-old recipient brain. (D) ES-cell-derived axons in the thalamus of a newborn recipient transplanted at E17. (Bars = 50 μm.)
Astrocytes generated by the transplanted ES cells were found in a distribution similar to that of donor-derived neurons. The most prominent accumulations were detected in the ventral diencephalon and in tectum. In addition, these cells efficiently incorporated into white matter regions such as the corpus callosum (Fig. 1). Donor-derived astrocytes strongly expressed M2, a species-specific antigen frequently used for the identification of mouse astrocytes in xenografts (Fig. 4 A and B and ref. 21). Their astroglial identity was confirmed by double labeling with an antibody to GFAP of cells labeled with either the mouse satellite probe or the M2 antibody (Fig. 4B). Encountered only occasionally in newborn recipients, these cells were more frequently detected in animals sacrificed at P15 (Table 1). ES-cell-derived astrocytes were morphologically indistinguishable from their host counterparts, and GFAP immunofluorescence showed no differences in size or branching pattern between the two populations. Typical for astroglia, donor-derived astrocytes often extended processes to adjacent blood vessels.
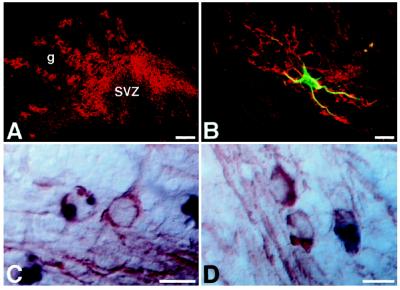
Figure 4.
Incorporation of ES-cell-derived glia. (A and B) ES-cell-derived astrocytes have migrated into the granular layer (g) of the olfactory bulb of a 2-week-old host. Cells are visualized with an antibody to the mouse-specific antigen M2 (red). SVZ, olfactory subventricular zone. An individual astrocyte, double labeled with an antibody to GFAP (green), is shown in B. (C and D) ES-cell-derived oligodendrocytes in the rostral (C) and caudal (D) corpus callosum of a 2-week-old host brain. The donor cells, identified by DNA in situ hybridization (black), are morphologically indistinguishable from adjacent host oligodendrocytes. Host- and donor-derived oligodendrocytes exhibit equivalent immunoreactivity to CNPase (red). [Bars = 100 μm (A) and 10 μm (B–D).]
In addition to neurons and astrocytes, donor-derived oligodendrocytes were found in the transplanted rat brains. The identity of these cells was confirmed by in situ hybridization with the mouse satellite probe and subsequent immunohistochemical detection of CNPase, a marker for myelin and oligodendroglia (22). ES-cell-derived CNPase-positive oligodendrocytes were only detected in the brains of 2-week survivors and their distribution was restricted to white matter regions such as the corpus callosum and striatal fiber tracts. Size, orientation, and CNPase expression of these cells were indistinguishable from their host counterparts (Fig. 4 C and D).
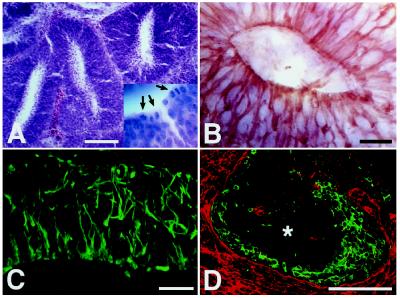
Formation of Mitotic Neuroepithelium.
Cells remaining in the host ventricle formed large clusters containing prominent neuroepithelial formations. These formations were particularly prominent in the 2-week survivors and—upon cross-sectioning—closely resembled tightly packed neural tubes (Fig. 5A). Individual tubes consisted of columnar epithelium with high mitotic activity at the luminal surface (Fig. 5A Inset). The epithelial cells showed strong expression of nestin (Fig. 5 B and C) and brain fatty-acid-binding protein (data not shown), antigens typically expressed in the early neuroepithelium (16, 23, 24). Cells expressing neuronal antigens, including microtubule-associated protein 2, neurofilament, and tyrosine hydroxylase, were restricted to the periphery of the formations, suggesting an inside-out gradient of differentiation (Fig. 5D). Thus, by morphology, antigen expression, and gradients of proliferation and differentiation, these structures are very similar to the developing neuroepithelium. In addition, intraventricular grafts contained small clusters of still undifferentiated embryonic cells. These cells were easily recognized by their expression of alkaline phosphatase, an enzyme typically present in undifferentiated embryonic cells (25). Whereas clusters of alkaline phosphatase-positive cells were frequently encountered in newborn animals, they were only occasionally detected in the 2-week survivors, arguing for a gradual differentiation of the embryonic cells. Disappearance of the alkaline phosphatase-positive cells was inversely correlated with the emergence of nonneural tissue in the P15 animals, where occasional islands containing adenoid structures, cartilage, and epidermis were found within the intraventricular clusters (Table 1). These observations indicate that undifferentiated embryonic cells present in the transplanted cell suspension form intraventricular teratomas.
Figure 5.
Generation of neuroepithelial formations. (A and B) Eight days after intrauterine transplantation, the donor cells have generated numerous neural tube-like structures within the host ventricle. Like the developing neural tube, these structures exhibit high mitotic activity at the luminal surface (A) (hematoxylin/eosin; arrows in Inset indicate mitotic figures) and strong expression of the intermediate filament nestin (B). (C and D) Neuroepithelial formation in the ventricle of a 2-week-old animal transplanted at E18. The formation contains abundant radially oriented nestin-positive processes (C). As in the early neuroepithelium, there is an inside-out gradient of differentiation with neuronal markers being expressed at the periphery of the formation (D) (green, tyrosine hydroxylase; red, M6; ∗, center of formation). [Bars = 100 μm (A and D) and 20 μm (B and C).]
DISCUSSION
Our results demonstrate that ES-cell-derived neural precursors transplanted into the developing mammalian brain are able to generate neuronal and glial lineages. Upon injection into the telencephalic vesicle of fetal rats, the donor cells leave the ventricle and migrate into the recipient brain, where they differentiate into neurons, astrocytes, and oligodendrocytes. Incorporation of the transplanted cells is not random but occurs in patterns compatible with the host brain development. The donor cells integrated preferentially into cortex, hippocampus, striatum, septum, the medial diencephalic nuclei, and tectum. These regions are known to continue neurogenesis until or even beyond the time of transplantation (26). Temporally, neurons, astrocytes, and oligodendrocytes appeared successively. Few astrocytes were present neonatally when compared with 2-week-old recipients (Table 1), and oligodendrocytes were only detected in the P15 animals. This delayed appearance of glial cells corresponds well with the timing of host gliogenesis that has been shown to be primarily a postnatal event (27). In addition, neurons and glia showed differences in their distribution. Although neurons incorporated preferentially into gray matter regions, astrocytes invaded both gray and white matter, and oligodendrocytes were found only in fiber tracts. These observations indicate that neural precursors derived from ES cells and transplanted into the mammalian brain are susceptible to environmental cues guiding cell fate determination and differential migration. Furthermore, donor-derived neurons appear to respond to local differentiation cues. This is particularly evident in cortex, where they acquired morphological features characteristic for cortical projection neurons, including a pyramidal cell body, long apical dendrites, and basal axons projecting into the corpus callosum (Fig. 2 B–D). The innervation of the host brain by the transplanted cells further suggests that donor-derived neurons remain responsive to host-mediated axon guidance. Detectable at all levels of the recipient brain, donor-derived axons bundled and extended through the major host trajectories, running alongside endogenous axons and obeying the border of the fiber tracts (Fig. 3C). Donor-derived astrocytes and oligodendrocytes were similarly indistinguishable from their endogenous counterparts and only detectable by virtue of their genetic difference. Donor and host glia exhibited equivalent expression of the cell-type-specific antigens GFAP and CNPase. Many of the ES-cell-derived astrocytes assumed perivascular locations, with one or several processes extending to the capillary wall—a feature typically observed in astrocytes involved in blood–brain barrier formation (data not shown).
The migration and differentiation patterns of the transplanted ES cells are in accordance with those obtained after intrauterine transplantation of primary cells derived from the embryonic brain (9–11). In both cases, the donor cells incorporate preferentially into regions exhibiting protracted neurogenesis until late gestation and adopt local phenotypic features. These observations indicate that primary neuroepithelial cells and neural precursors derived from ES cells respond very similarly to environmental cues. Our data strongly suggest that neuroepithelial cells generated in vitro from ES cells can act as neural precursors in vivo and contribute neurons and glia to the developing mammalian brain. These findings significantly extend results of previous studies showing that retinoic acid-induced ES cells exhibit antigenic and electrophysiological properties of neurons and glia in vitro (4–7) and that the differentiated phenotype of retinoic acid-induced ES or teratocarcinoma cells can be maintained after transplantation into the adult brain (28–32). In contrast to these studies, we were less interested in the forceful induction and subsequent maintenance of a neuronal phenotype but rather in the interaction of still undifferentiated neural precursors with the developing brain. The widespread neuronal and glial integration obtained after transplantation of ES-cell-derived precursors provides an impressive example for the dominant role of non-cell-autonomous signals during neural migration and differentiation. The fact that a cell never previously exposed to a nervous system is able to migrate into cortex and to differentiate into an appropriate local phenotype illustrates that cell communities in individual brain regions harbor sufficient cues to maintain their local identity and to foster their own development through precursor cell recruitment.
It is a well-described phenomenon that ES cells transplanted to an adult host frequently develop teratomas and teratocarcinomas (for review, see ref. 33). In striking contrast, both ES and teratocarcinoma cells have been shown to participate in normal development upon introduction into early embryos at the blastocyst stage (for review, see ref. 34). Our observations, along with similar studies on ES-cell-derived hematopoietic progenitors (3), provide an interesting intermediate between these two scenarios. They show that the ability to participate in normal development is not restricted to undifferentiated ES cells but extends to their more differentiated progeny. Neural and hematopoietic precursors derived from ES cells in vitro are able to reconstitute neural and hematopoietic lineages after transplantation into living hosts. It is likely that such a contribution to host tissue formation requires close physical contact between the transplanted cells and the target tissue. One explanation for the conspicuous formation of neural tube-like structures within the host ventricles might be that ES cells differentiated into neural precursors but physically separated from the brain tissue are not sufficiently exposed to local cues mediating precursor cell recruitment and thus develop autonomously into primitive nervous system tissue. ES cells not sufficiently differentiated into neural precursors might evade recruitment because they are unable to respond to tissue-specific guidance cues. Because of their pluripotency, these cells can develop into a variety of tissues. Islands of nonneural tissue observed within the intraventricular clusters are most likely derivatives of still undifferentiated ES cells present in the transplanted cell suspension.
Although our results outline the developmental potential of in vitro-generated neural precursors at a basic neurobiological level, the in vivo reconstitution of neuronal and glial lineages by transplanted ES-cell-derived donor cells might eventually be exploited for cell replacement strategies. This idea receives strong impetus from recent findings implying that embryonic stem cells can be obtained from adult tissue by transferring nuclei of differentiated cells into oocytes (35), a perspective offering the possibility to generate virtually unlimited numbers of tissue-specific and genetically modified donor cells from the same individual. Transplant experiments in rodents further indicate that the adult brain may retain some of the cues required for regional cellular differentiation (36–38). These observations would suggest that region-specific differentiation of transplanted ES-cell-derived precursors is not limited to the developing nervous system. The feasibility of ES-cell-based replacement strategies will critically depend on the ability to generate highly purified donor populations susceptible to host regulation.
The possibility of introducing ES-cell-derived neurons and glia into the developing nervous system also offers an exciting approach for the study of neurological mutants. The rapid proliferation of ES cells and their susceptibility to genetic manipulation allows the generation of large numbers of genetically modified donor cells. The properties of these cells can then be assayed in vivo in a wild-type recipient brain. The data presented herein show that the full range of neuronal and glial phenotypes might be accessible with this approach. Analysis is not restricted to cell migration and differentiation but may include aspects such as axon outgrowth and guidance, myelination, and the susceptibility of distinct genotypes to degenerative and neoplastic brain disease. Transplantation of ES-cell-derived neural precursors should be especially useful for the analysis of targeted gene deletions. Homozygous knockout ES cells from mutants exhibiting very early embryonic lethality can be differentiated in vitro and their neural offspring can be analyzed in the context of a developing brain. Incorporation of ES-cell-derived precursors into the central nervous system also offers the possibility to analyze the neural phenotype of null mutants without prior generation of a knockout animal.
Acknowledgments
We thank Carl Lagenaur for providing the M6 and M2 antibodies. We gratefully acknowledge Kim Jones and Robert Green for help with the tissue processing, Carolyn Smith for her advice on confocal laser microscopy, and Tom Hazel for his helpful suggestions.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: E, embryonic day; ES cell, embryonic stem cell; P, postnatal day; GFAP, glial fibrillary acidic protein; CNPase, 2′,3′-cyclic nucleotide 3′-phosphodiesterase.
References
- 1.Evans M J, Kaufman M H. Nature (London) 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin G R. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palacios R, Golunski E, Samaridis J. Proc Natl Acad Sci USA. 1995;92:7530–7534. doi: 10.1073/pnas.92.16.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain G, Kitchens D, Yao M, Huettner J E, Gottlieb D I. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 5.Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. J Cell Sci. 1995;108:3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- 6.Strübing C, Ahnert-Hilger G, Shan J, Wiedenmann B, Hescheler J, Wobus A M. Mech Dev. 1995;53:275–287. doi: 10.1016/0925-4773(95)00446-8. [DOI] [PubMed] [Google Scholar]
- 7.Finley M F A, Kulkarni N, Huettner J E. J Neurosci. 1996;16:1056–1065. doi: 10.1523/JNEUROSCI.16-03-01056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okabe S, Forsberg-Nilsson K, Spiro A C, Segal M, McKay R D G. Mech Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 9.Fishell G. Development (Cambridge, UK) 1995;121:803–812. doi: 10.1242/dev.121.3.803. [DOI] [PubMed] [Google Scholar]
- 10.Campbell K, Olsson M, Björklund A. Neuron. 1995;15:1259–1273. doi: 10.1016/0896-6273(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 11.Brüstle O, Maskos U, McKay R D G. Neuron. 1995;15:1275–1285. doi: 10.1016/0896-6273(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 12.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 13.Lagenaur C, Schachner M. J Supramol Struct Cell Biochem. 1981;15:335–346. doi: 10.1002/jsscb.1981.380150404. [DOI] [PubMed] [Google Scholar]
- 14.Lund R D, Chang F L F, Hankin M H, Lagenaur C F. Neurosci Lett. 1985;61:221–226. doi: 10.1016/0304-3940(85)90428-8. [DOI] [PubMed] [Google Scholar]
- 15.Hörz W, Altenburger W. Nucleic Acids Res. 1981;9:683–696. doi: 10.1093/nar/9.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lendahl U, Zimmermann L B, McKay R D G. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 17.Solter D, Knowles B B. Proc Natl Acad Sci USA. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemler R, Brûlet P, Schnebelen M T, Gaillard J, Jacob F. J Embryol Exp Morphol. 1981;64:45–60. [PubMed] [Google Scholar]
- 19.Mullen R J, Buck C R, Smith A M. Development (Cambridge, UK) 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 20.de Lorente N-R. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- 21.Zhou H F, Lee L H C, Lund R D. J Comp Neurol. 1990;292:320–330. doi: 10.1002/cne.902920213. [DOI] [PubMed] [Google Scholar]
- 22.Sprinkle T J. CRC Crit Rev Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- 23.Frederiksen K, McKay R D G. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtz A, Zimmer A, Schnütgen F, Brüning G, Spener F, Müller T. Development (Cambridge, UK) 1994;120:2637–2649. doi: 10.1242/dev.120.9.2637. [DOI] [PubMed] [Google Scholar]
- 25.Bernstine E G, Hooper M L, Grandchamp S, Ephrussi B. Proc Natl Acad Sci USA. 1973;70:3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altman J, Bayer S A. Atlas of Prenatal Rat Brain Development. Boca Raton, FL: CRC; 1995. [Google Scholar]
- 27.Levison S W, Chuang C, Abramson B J, Goldman J E. Development (Cambridge, UK) 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- 28.Trojanowski J Q, Mantione J R, Lee J H, Seid D P, You T, Inge L J, Lee V M Y. Exp Neurol. 1993;122:283–294. doi: 10.1006/exnr.1993.1128. [DOI] [PubMed] [Google Scholar]
- 29.Wojcik B E, Nothias F, Lazar M, Jouin H, Nicolas J F, Peschanski M. Proc Natl Acad Sci USA. 1993;90:1305–1309. doi: 10.1073/pnas.90.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morassutti D J, Staines W A, Magnuson D S K, Marshall K C, McBurney M W. Neuroscience. 1994;58:753–763. doi: 10.1016/0306-4522(94)90452-9. [DOI] [PubMed] [Google Scholar]
- 31.Magnuson D S, Morassutti D J, Staines W A, McBurney M W, Marshall K C. Brain Res Dev Brain Res. 1995;84:130–141. doi: 10.1016/0165-3806(94)00166-w. [DOI] [PubMed] [Google Scholar]
- 32.Dinsmore J, Ratliff J, Deacon T, Pakzaban P, Jacoby D, Galpern W, Isacson O. Cell Transplant. 1996;5:131–143. doi: 10.1177/096368979600500205. [DOI] [PubMed] [Google Scholar]
- 33.Damjanov I. Int J Dev Biol. 1993;37:39–46. [PubMed] [Google Scholar]
- 34.Brinster R L. Int J Dev Biol. 1993;37:89–99. [PubMed] [Google Scholar]
- 35.Wilmut I, Schnieke A E, McWhir J, Kind A J, Campbell K H S. Nature (London) 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 36.Shihabuddin L S, Hertz J A, Holets V R, Whittemore S R. J Neurosci. 1995;15:6666–6678. doi: 10.1523/JNEUROSCI.15-10-06666.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheen V L, Macklis J D. J Neurosci. 1995;15:8378–8392. doi: 10.1523/JNEUROSCI.15-12-08378.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suhonen J O, Peterson D A, Ray J, Gage F H. Nature (London) 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]