Abstract
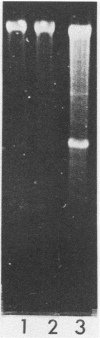
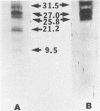
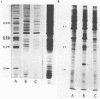
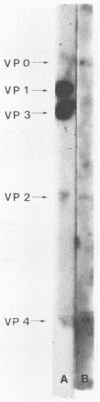
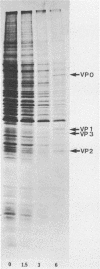
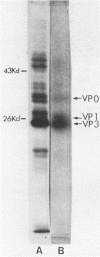
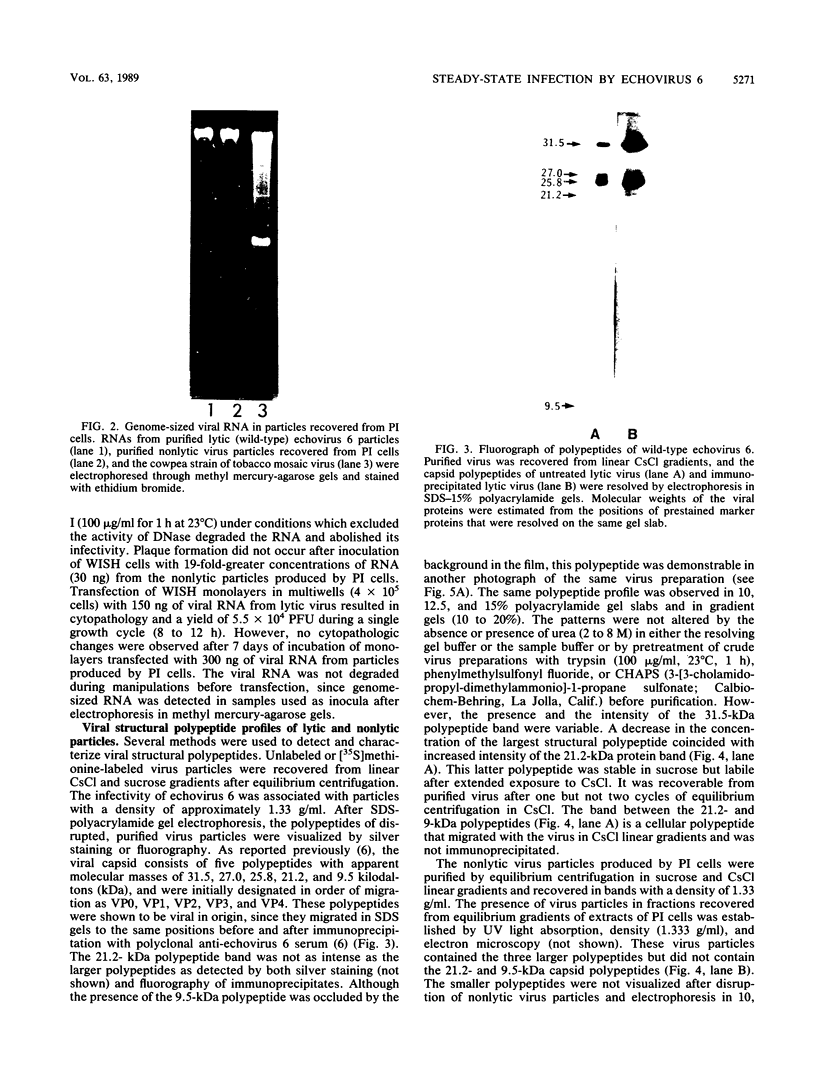
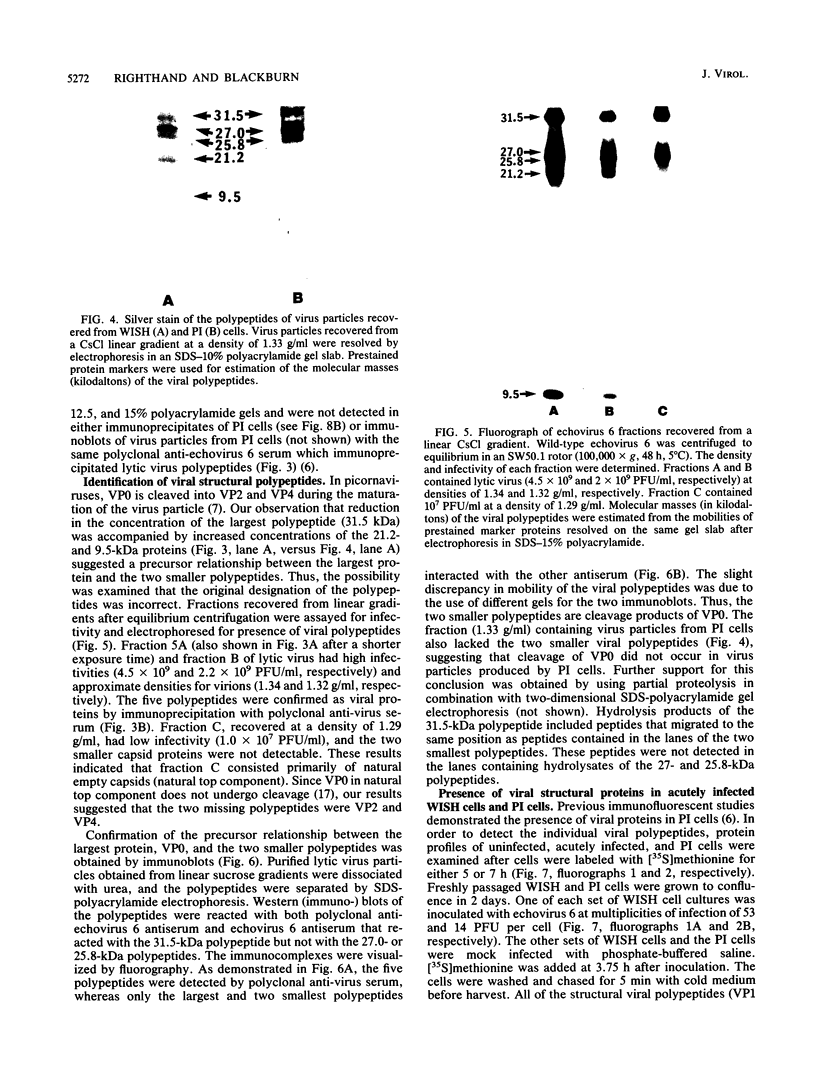
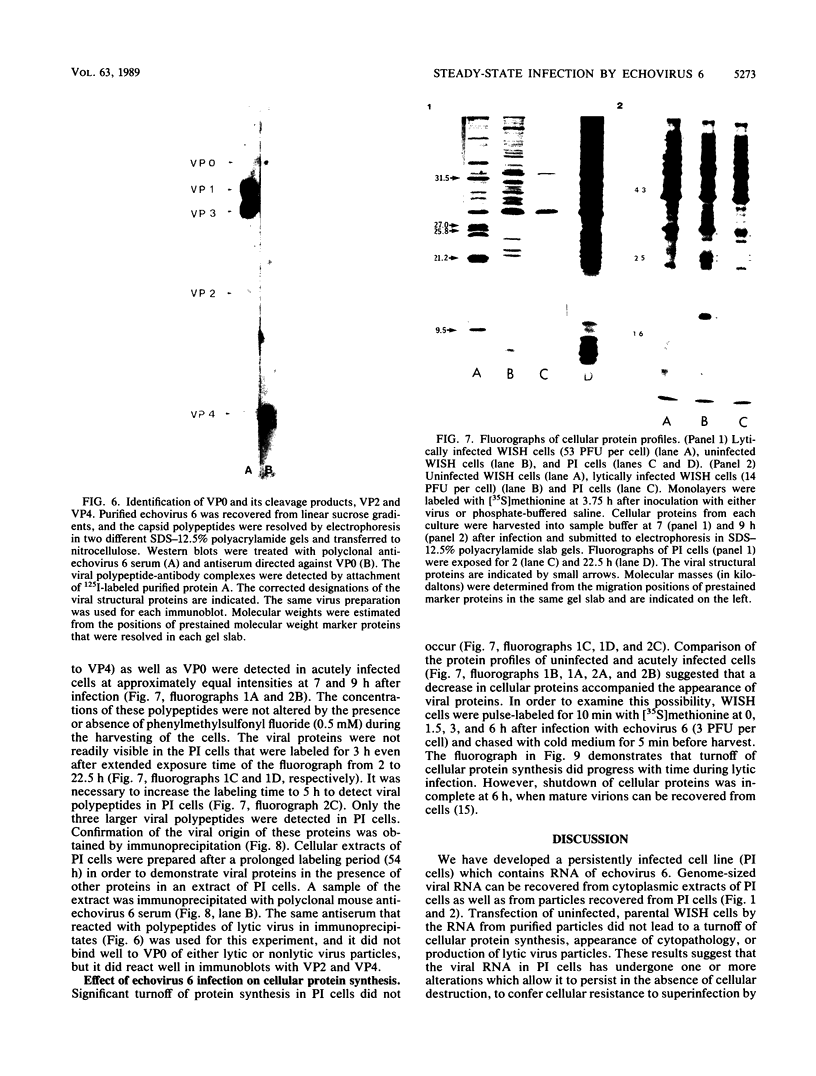
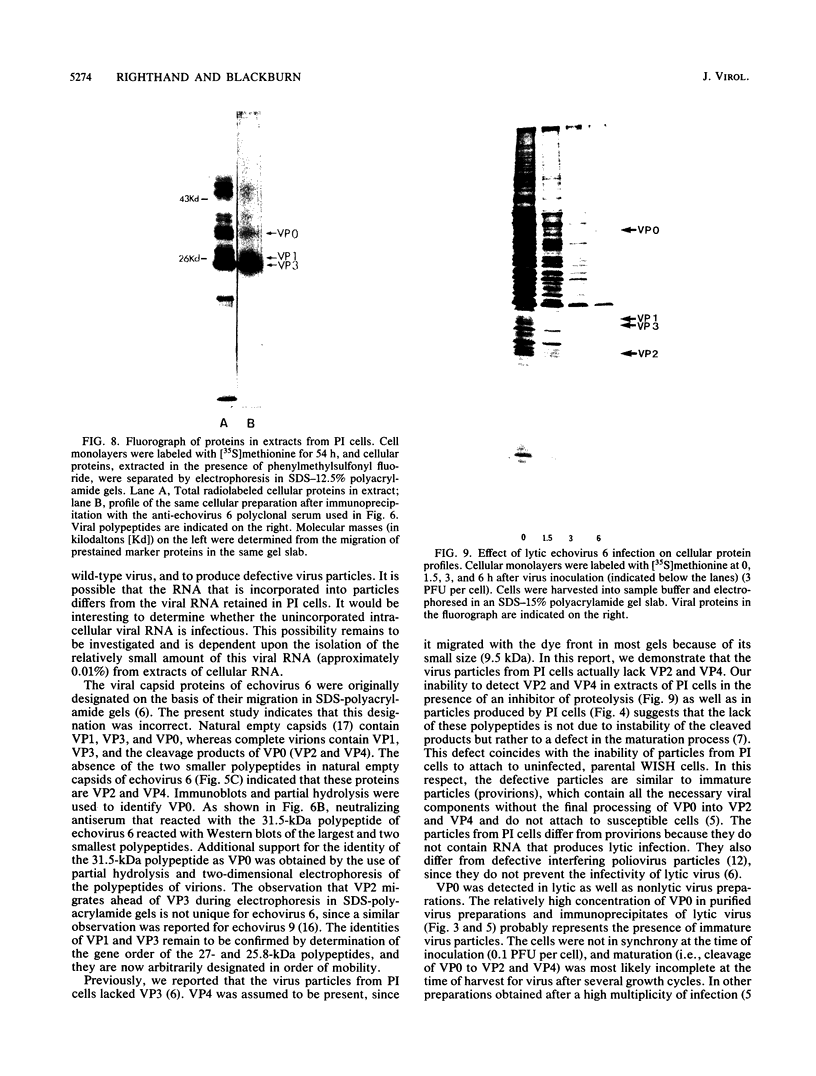
We established a human cell line which was persistently infected (PI) by the normally cytolytic echovirus 6. All of the cultured PI cells contained genome-size viral RNA which was synthesized continuously and incorporated into virus particles. This steady-state infection has been maintained for more than 6 years. In contrast to RNA of wild-type echovirus 6, the viral RNA from PI cells was not lytic when transfected into uninfected, susceptible cells. The capsid polypeptides of the virus particles produced during lytic infections were compared with those of virus particles from PI cells. Wild-type virions contained five polypeptides with molecular masses of 31.5, 27, 25.8, 21.2, and 9.5 kilodaltons. Comparison of polypeptide profiles of virions and empty immature capsids along with peptide analyses by immunoblotting and partial proteolysis of isolated viral proteins identified the cleavage products of the 31.5-kilodalton polypeptide (VP0) as the two smaller polypeptides (VP2 and VP4). The virus particles produced by PI cells as well as cellular extracts of PI cells contained only the three largest proteins (VP0, VP1, and VP3), indicating that VP0 was not processed during persistent infection. The lack of VP2 and VP4 in the defective virus particles coincided with their inability to attach to uninfected, susceptible cells. The maintenance of the steady-state infection of echovirus 6 was not dependent upon the release of virus particles from PI cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRON A. L., KARZON D. T. STUDIES OF MUTANTS OF ECHOVIRUS 6. I. BIOLOGIC AND SEROLOGIC CHARACTERISTICS. Am J Epidemiol. 1965 May;81:323–332. doi: 10.1093/oxfordjournals.aje.a120518. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Baltimore D. Morphogenesis of poliovirus. II. Demonstration of a new intermediate, the proviron. J Virol. 1973 Nov;12(5):1122–1130. doi: 10.1128/jvi.12.5.1122-1130.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. P., Righthand V. F. Persistence of echovirus 6 in cloned human cells. J Virol. 1985 Apr;54(1):219–223. doi: 10.1128/jvi.54.1.219-223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Levy A., Racaniello V. R. Isolation and characterization of HeLa cell lines blocked at different steps in the poliovirus life cycle. J Virol. 1989 Jan;63(1):43–51. doi: 10.1128/jvi.63.1.43-51.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Holland J. J., Perrault J. Generation of defective interfering particles in picornaviruses. Virology. 1980 Jan 30;100(2):408–418. doi: 10.1016/0042-6822(80)90532-2. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Righthand F., Karzon D. T. Effect of enhancer on growth cycle of ECHO virus 6 plaque mutant. Arch Gesamte Virusforsch. 1969;27(2):290–300. doi: 10.1007/BF01249651. [DOI] [PubMed] [Google Scholar]
- Righthand V. F., Hughes P. F. Isolation of a specific enhancer for echovirus 6 from uninfected permissive host cells. Infect Immun. 1974 Jan;9(1):134–141. doi: 10.1128/iai.9.1.134-141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwirth B., Eggers H. J. Biochemistry and pathogenicity of echovirus 9. I. Characterization of the virus particles of strains Barty and Hill. Virology. 1982 Nov;123(1):102–112. doi: 10.1016/0042-6822(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Dávila M., Sobrino F., Ortín J., Domingo E. Establishment of cell lines persistently infected with foot-and-mouth disease virus. Virology. 1985 Aug;145(1):24–35. doi: 10.1016/0042-6822(85)90198-9. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Diez J., Villaverde A., Gebauer F., Rocha E., Dávila M., Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988 Jun;62(6):2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]