Abstract
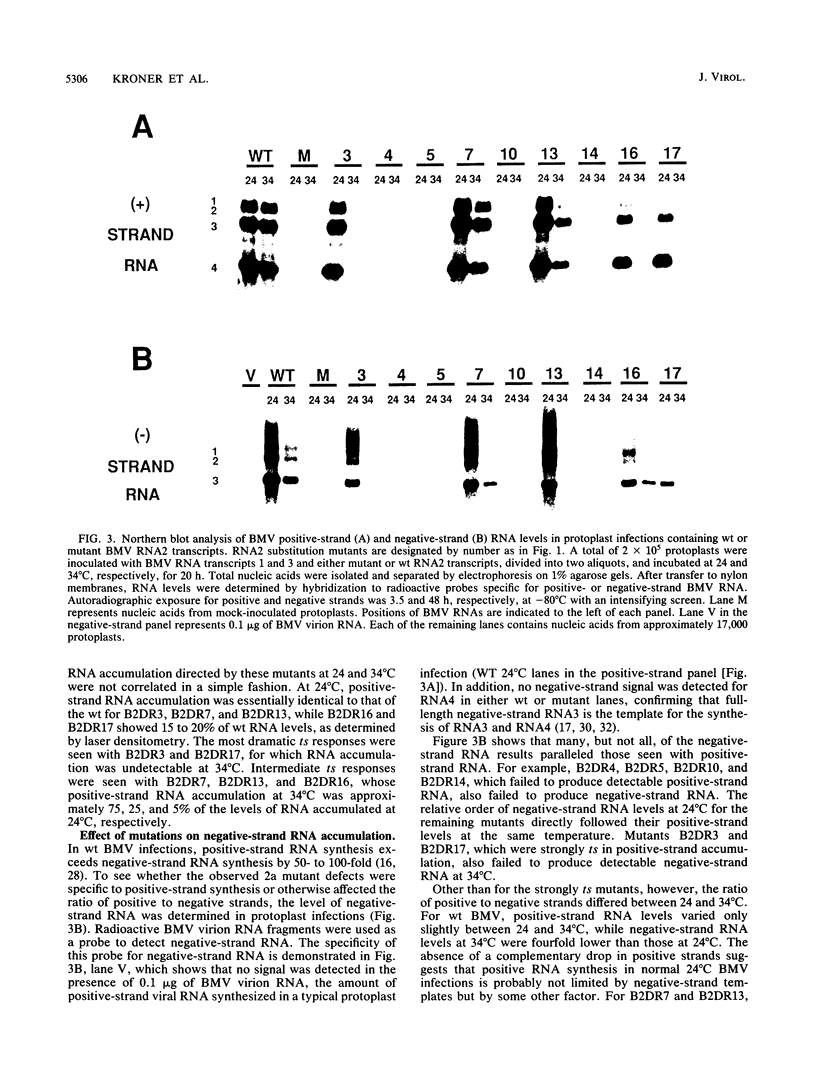
The central portion of the brome mosaic virus (BMV) 2a protein represents the most conserved element among the related RNA replication components of a large group of positive-strand RNA viruses of humans, animals, and plants. To characterize the functions of the 2a protein, mutations were targeted to a conserved portion of the 2a gene, resulting in substitutions between amino acids 451 and 484. After the temperature profile of wild-type BMV RNA replication was defined, RNA replication by nine selected mutants was tested in barley protoplasts at permissive (24 degrees C) and nonpermissive (34 degrees C) temperatures. Four mutants did not direct RNA synthesis at either temperature. Various levels of temperature-sensitive (ts) replication occurred in the remaining five mutants. For two ts mutants, no viral RNA synthesis was detected at 34 degrees C, while for two others, an equivalent reduction in positive- and negative-strand RNA accumulation was observed. For one mutant, positive-strand accumulation was preferentially reduced over negative-strand accumulation at 34 degrees C. Moreover, this mutant and another displayed preferential suppression of genomic over subgenomic RNA accumulation at both 24 and 34 degrees C. The combination of phenotypes observed suggests that the 2a protein may play a role in the differential initiation of specific classes of viral RNA in addition to a previously suggested role in RNA elongation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Nucleotide sequence of the brome mosaic virus genome and its implications for viral replication. J Mol Biol. 1984 Feb 5;172(4):369–383. doi: 10.1016/s0022-2836(84)80012-1. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., French R., Janda M., Loesch-Fries L. S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist P., Luckow V., Kaesberg P. Complete nucleotide sequence of brome mosaic virus RNA3. J Mol Biol. 1981 Nov 25;153(1):23–38. doi: 10.1016/0022-2836(81)90524-6. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Strauss E. G., Rice C. M., Strauss J. H., Haseloff J., Zimmern D. Sindbis virus proteins nsP1 and nsP2 contain homology to nonstructural proteins from several RNA plant viruses. J Virol. 1985 Feb;53(2):536–542. doi: 10.1128/jvi.53.2.536-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P. A sequence motif in many polymerases. Nucleic Acids Res. 1988 Nov 11;16(21):9909–9916. doi: 10.1093/nar/16.21.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D. J., Sawicki S. G., Sawicki D. L. Demonstration in vitro of temperature-sensitive elongation of RNA in Sindbis virus mutant ts6. J Virol. 1988 Oct;62(10):3597–3602. doi: 10.1128/jvi.62.10.3597-3602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Carmichael G. G. RNA replication: function and structure of Qbeta-replicase. Annu Rev Biochem. 1979;48:525–548. doi: 10.1146/annurev.bi.48.070179.002521. [DOI] [PubMed] [Google Scholar]
- Bujarski J. J., Ahlquist P., Hall T. C., Dreher T. W., Kaesberg P. Modulation of replication, aminoacylation and adenylation in vitro and infectivity in vivo of BMV RNAs containing deletions within the multifunctional 3' end. EMBO J. 1986 Aug;5(8):1769–1774. doi: 10.1002/j.1460-2075.1986.tb04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski J. J., Dreher T. W., Hall T. C. Deletions in the 3'-terminal tRNA-like structure of brome mosaic virus RNA differentially affect aminoacylation and replication in vitro. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5636–5640. doi: 10.1073/pnas.82.17.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., White J. L. A temperature-sensitive mutant of tobacco mosaic virus deficient in synthesis of single-stranded RNA. Virology. 1979 Feb;93(1):104–110. doi: 10.1016/0042-6822(79)90279-4. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., White J. L. Characterization of a temperature-sensitive mutant of tobacco mosaic virus deficient in synthesis of all RNA species. Virology. 1978 Oct 15;90(2):209–213. doi: 10.1016/0042-6822(78)90304-5. [DOI] [PubMed] [Google Scholar]
- French R., Ahlquist P. Characterization and engineering of sequences controlling in vivo synthesis of brome mosaic virus subgenomic RNA. J Virol. 1988 Jul;62(7):2411–2420. doi: 10.1128/jvi.62.7.2411-2420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Ahlquist P. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J Virol. 1987 May;61(5):1457–1465. doi: 10.1128/jvi.61.5.1457-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. Genome similarities between plant and animal RNA viruses. Microbiol Sci. 1987 Jul;4(7):197–202. [PubMed] [Google Scholar]
- Hahn Y. S., Grakoui A., Rice C. M., Strauss E. G., Strauss J. H. Mapping of RNA- temperature-sensitive mutants of Sindbis virus: complementation group F mutants have lesions in nsP4. J Virol. 1989 Mar;63(3):1194–1202. doi: 10.1128/jvi.63.3.1194-1202.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi Y., Hirashima A. Interference with viral infection by defective RNA replicase. J Virol. 1987 Dec;61(12):3946–3949. doi: 10.1128/jvi.61.12.3946-3949.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M., French R., Ahlquist P. High efficiency T7 polymerase synthesis of infectious RNA from cloned brome mosaic virus cdna and effects of 5' extensions on transcript infectivity. Virology. 1987 May;158(1):259–262. doi: 10.1016/0042-6822(87)90265-0. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiberstis P. A., Loesch-Fries L. S., Hall T. C. Viral protein synthesis in barley protoplasts inoculated with native and fractionated brome mosaic virus RNA. Virology. 1981 Jul 30;112(2):804–808. doi: 10.1016/0042-6822(81)90331-7. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. A., Dreher T. W., Hall T. C. Synthesis of brome mosaic virus subgenomic RNA in vitro by internal initiation on (-)-sense genomic RNA. Nature. 1985 Jan 3;313(5997):68–70. doi: 10.1038/313068a0. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Hall T. C. Use of micrococcal nuclease in the purification of highly template dependent RNA-dependent RNA polymerase from brome mosaic virus-infected barley. Virology. 1983 Feb;125(1):236–241. doi: 10.1016/0042-6822(83)90077-6. [DOI] [PubMed] [Google Scholar]
- Mills D. R., Priano C., DiMauro P., Binderow B. D. Q beta replicase: mapping the functional domains of an RNA-dependent RNA polymerase. J Mol Biol. 1989 Feb 20;205(4):751–764. doi: 10.1016/0022-2836(89)90319-7. [DOI] [PubMed] [Google Scholar]
- Nassuth A., Bol J. F. Altered balance of the synthesis of plus- and minus-strand RNAs induced by RNAs 1 and 2 of alfalfa mosaic virus in the absence of RNA 3. Virology. 1983 Jan 15;124(1):75–85. doi: 10.1016/0042-6822(83)90291-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Quadt R., Verbeek H. J., Jaspars E. M. Involvement of a nonstructural protein in the RNA synthesis of brome mosaic virus. Virology. 1988 Jul;165(1):256–261. doi: 10.1016/0042-6822(88)90679-4. [DOI] [PubMed] [Google Scholar]
- Sacher R., French R., Ahlquist P. Hybrid brome mosaic virus RNAs express and are packaged in tobacco mosaic virus coat protein in vivo. Virology. 1988 Nov;167(1):15–24. doi: 10.1016/0042-6822(88)90049-9. [DOI] [PubMed] [Google Scholar]
- Samac D. A., Nelson S. E., Sue Loesch-Fries L. Virus protein synthesis in alfalfa mosaic virus infected alfalfa protoplasts. Virology. 1983 Dec;131(2):455–462. doi: 10.1016/0042-6822(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Sarachu A. N., Huisman M. J., van Vloten-Doting L., Bol J. F. Alfalfa mosaic virus temperature-sensitive mutants. I. Mutants defective in viral RNA and protein synthesis. Virology. 1985 Feb;141(1):14–22. doi: 10.1016/0042-6822(85)90178-3. [DOI] [PubMed] [Google Scholar]
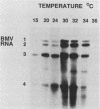
- Sawicki S. G., Sawicki D. L., Käriäinen L., Keränen S. A Sindbis virus mutant temperature-sensitive in the regulation of minus-strand RNA synthesis. Virology. 1981 Nov;115(1):161–172. doi: 10.1016/0042-6822(81)90098-2. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. A., Rickles R. J., Flanegan J. B. Genome-length copies of poliovirion RNA are synthesized in vitro by the poliovirus RNA-dependent RNA polymerase. J Biol Chem. 1982 Apr 25;257(8):4610–4617. [PubMed] [Google Scholar]