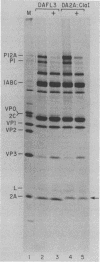
Abstract
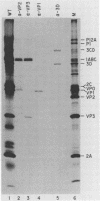
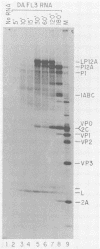
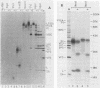
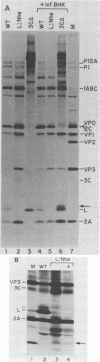
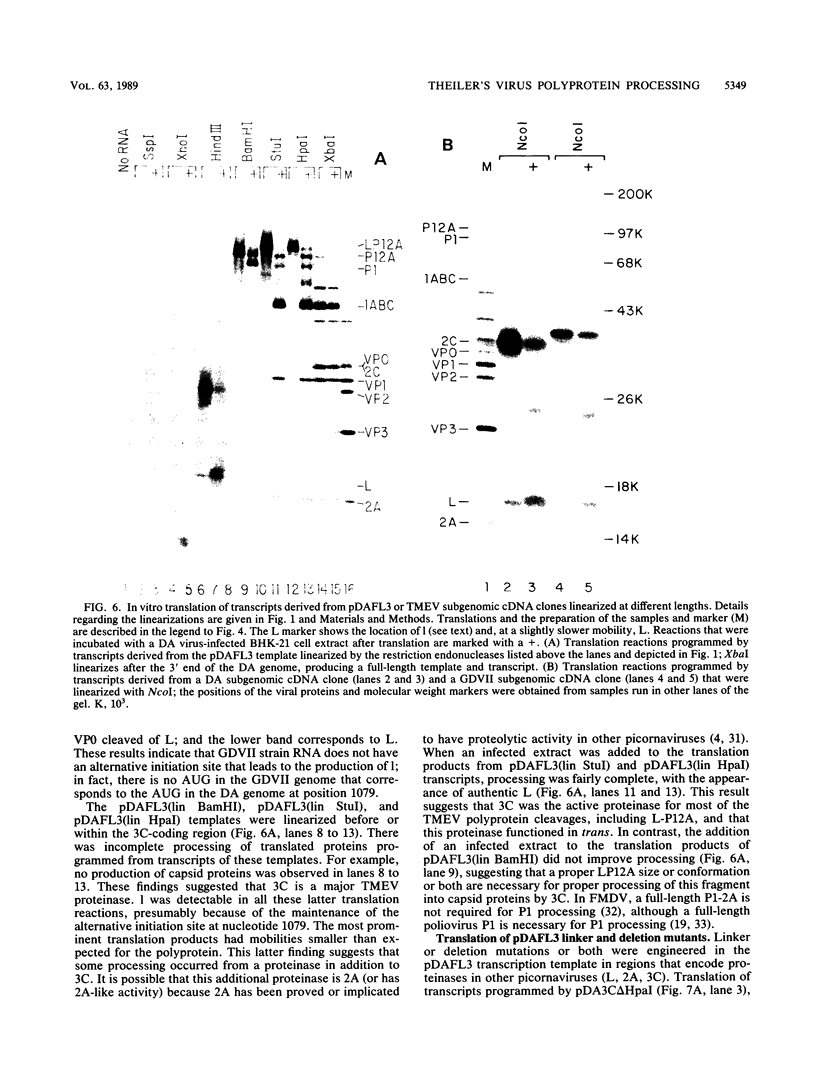
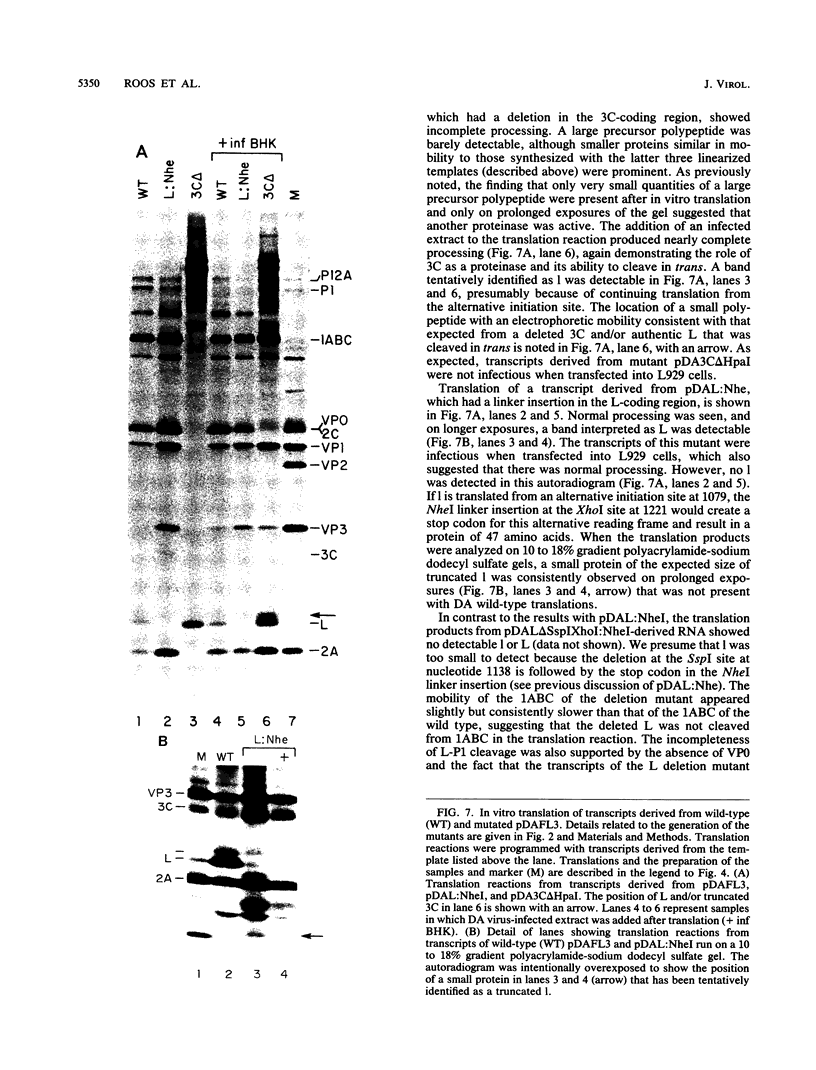
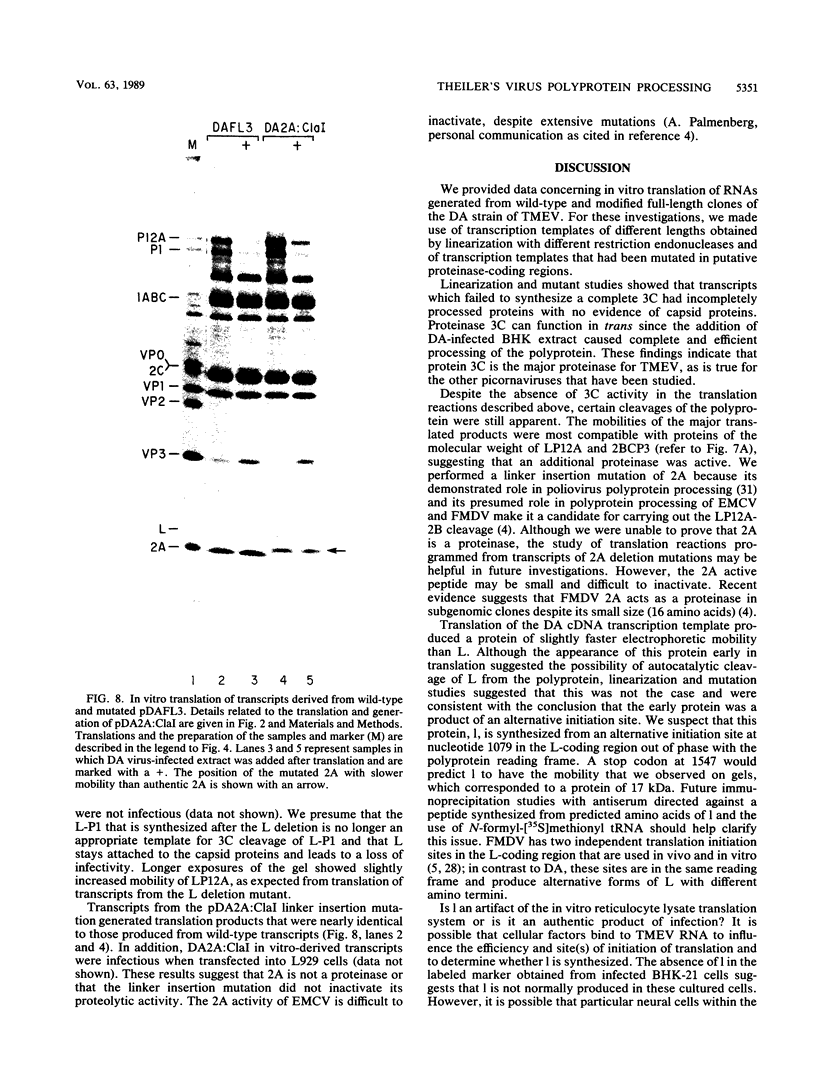
To investigate polyprotein processing of Theiler's murine encephalomyelitis viruses, we analyzed in vitro translation reactions programmed by in vitro-derived transcripts from an infectious full-length cDNA clone of the DA strain of Theiler's virus. To help identify the proteinases that carried out the processing, we modified the DA cDNA clone transcription template by linearization with different restriction endonucleases that generate templates of different lengths or by constructing linker insertion or deletion mutations or both in putative proteinase-coding regions. Protein 3C carried out most of the cleavages of the polyprotein, as is true for the other picornaviruses that have been studied. A second proteinase also appeared active at the LP12A-2B junction. A protein of slightly faster mobility than the leader protein was seen with translation of transcripts derived from DA cDNA but not GDVII cDNA. This protein may be synthesized from an alternative initiation site in the DA leader-coding region out of phase with the polyprotein reading frame. Our findings are relevant to ongoing investigations of the abnormal virus expression seen in DA virus late demyelinating disease, since polyprotein processing is critical in regulating picornaviral gene expression.
Full text
PDF
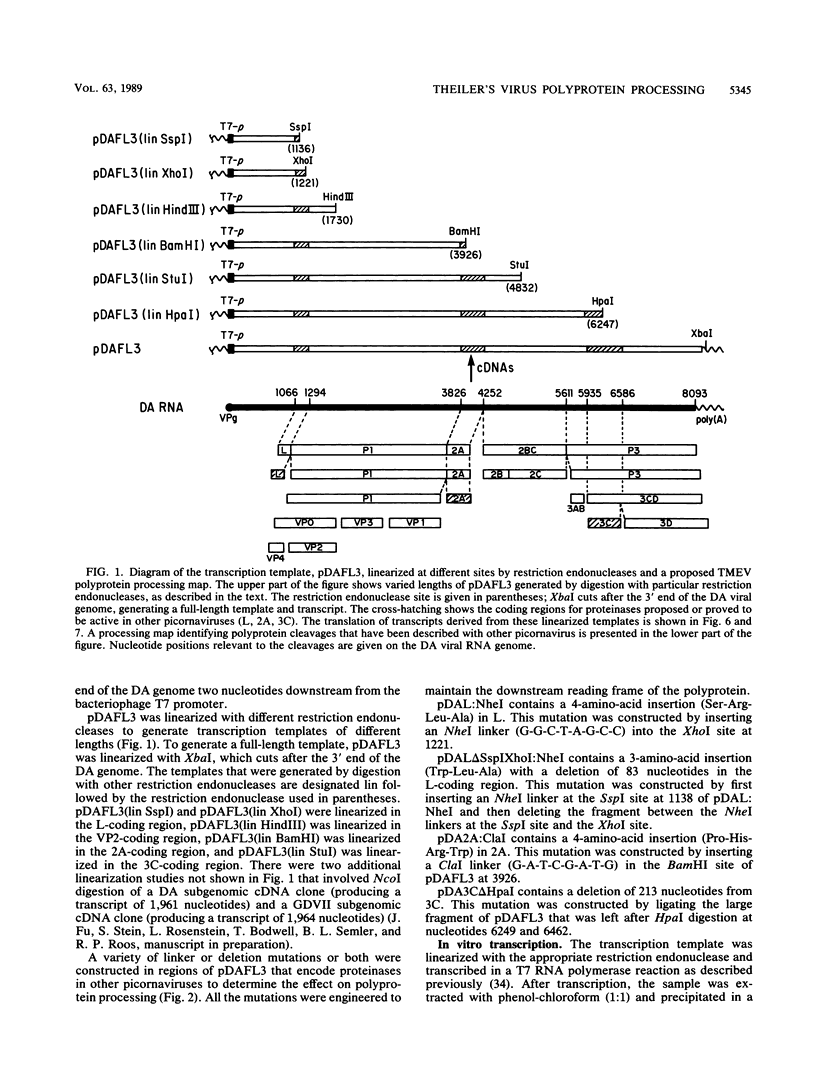
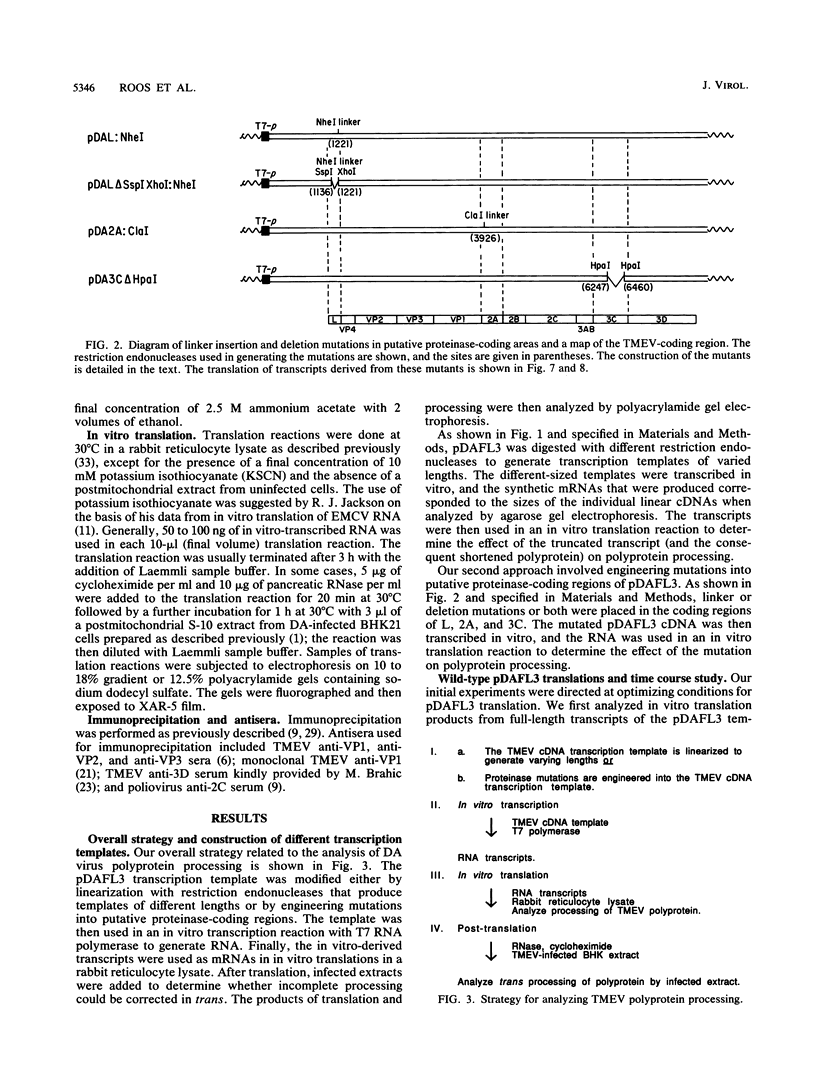
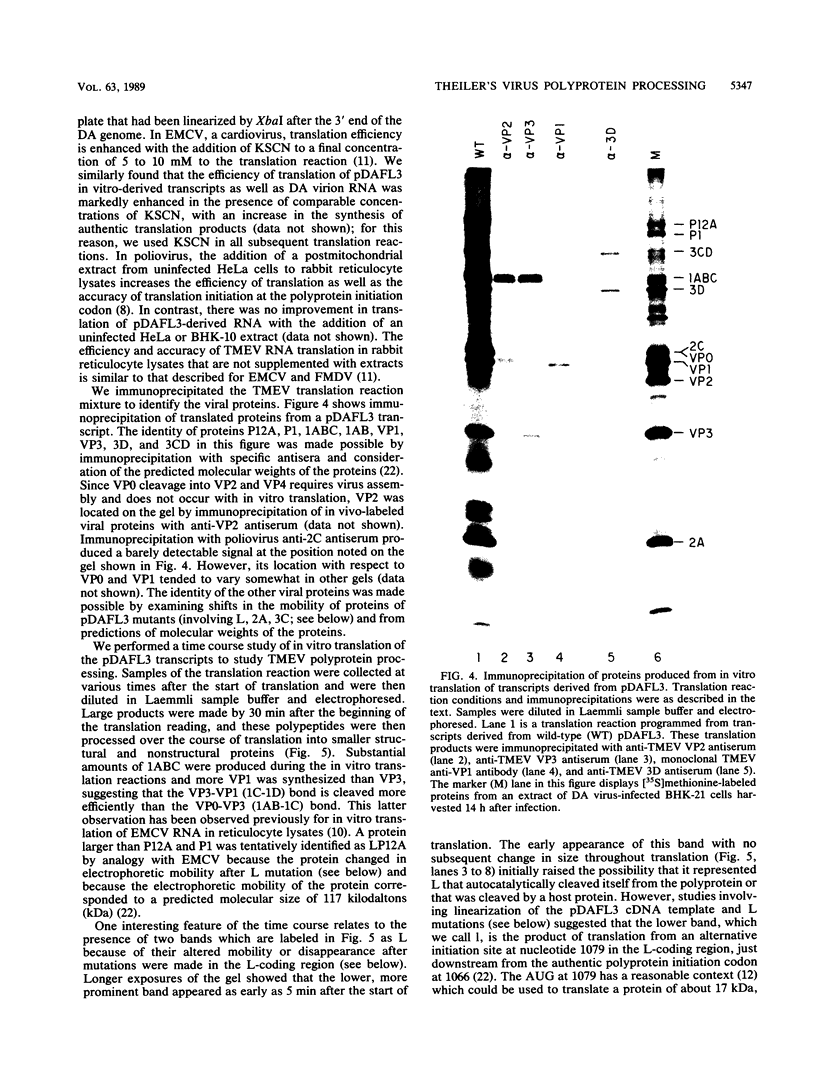



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown B. A., Ehrenfeld E. Translation of poliovirus RNA in vitro: changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology. 1979 Sep;97(2):396–405. doi: 10.1016/0042-6822(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Campbell E. A., Jackson R. J. Processing of the encephalomyocarditis virus capsid precursor protein studied in rabbit reticulocyte lysates incubated with N-formyl-[35S]methionine-tRNAfMet. J Virol. 1983 Jan;45(1):439–441. doi: 10.1128/jvi.45.1.439-441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash E., Chamorro M., Brahic M. Theiler's virus RNA and protein synthesis in the central nervous system of demyelinating mice. Virology. 1985 Jul 15;144(1):290–294. doi: 10.1016/0042-6822(85)90327-7. [DOI] [PubMed] [Google Scholar]
- Clarke B. E., Sangar D. V., Burroughs J. N., Newton S. E., Carroll A. R., Rowlands D. J. Two initiation sites for foot-and-mouth disease virus polyprotein in vivo. J Gen Virol. 1985 Dec;66(Pt 12):2615–2626. doi: 10.1099/0022-1317-66-12-2615. [DOI] [PubMed] [Google Scholar]
- Clarke B. E., Sangar D. V. Processing and assembly of foot-and-mouth disease virus proteins using subgenomic RNA. J Gen Virol. 1988 Sep;69(Pt 9):2313–2325. doi: 10.1099/0022-1317-69-9-2313. [DOI] [PubMed] [Google Scholar]
- Clatch R. J., Pevear D. C., Rozhon E., Roos R. P., Miller S. D., Lipton H. L. Characterization and specificity of humoral immune responses to Theiler's murine encephalomyelitis virus capsid proteins. J Gen Virol. 1987 Dec;68(Pt 12):3191–3196. doi: 10.1099/0022-1317-68-12-3191. [DOI] [PubMed] [Google Scholar]
- Devaney M. A., Vakharia V. N., Lloyd R. E., Ehrenfeld E., Grubman M. J. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J Virol. 1988 Nov;62(11):4407–4409. doi: 10.1128/jvi.62.11.4407-4409.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Semler B. L., Jackson R. J., Hanecak R., Duprey E., Wimmer E. In vitro translation of poliovirus RNA: utilization of internal initiation sites in reticulocyte lysate. J Virol. 1984 May;50(2):507–514. doi: 10.1128/jvi.50.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J. A detailed kinetic analysis of the in vitro synthesis and processing of encephalomyocarditis virus products. Virology. 1986 Feb;149(1):114–127. doi: 10.1016/0042-6822(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Persistent Theiler's murine encephalomyelitis virus infection in mice depends on plaque size. J Gen Virol. 1980 Jan;46(1):169–177. doi: 10.1099/0022-1317-46-1-169. [DOI] [PubMed] [Google Scholar]
- Lloyd R. E., Grubman M. J., Ehrenfeld E. Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J Virol. 1988 Nov;62(11):4216–4223. doi: 10.1128/jvi.62.11.4216-4223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch Y., Friedmann A., Lipton H. L., Kotler M. Theiler's murine encephalomyelitis virus group includes two distinct genetic subgroups that differ pathologically and biologically. J Virol. 1981 Nov;40(2):560–567. doi: 10.1128/jvi.40.2.560-567.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosenkis J., Daniels-McQueen S., Janovec S., Duncan R., Hershey J. W., Grifo J. A., Merrick W. C., Thach R. E. Shutoff of host translation by encephalomyocarditis virus infection does not involve cleavage of the eucaryotic initiation factor 4F polypeptide that accompanies poliovirus infection. J Virol. 1985 May;54(2):643–645. doi: 10.1128/jvi.54.2.643-645.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Kräusslich H. G., Toyoda H., Dunn J. J., Wimmer E. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4002–4006. doi: 10.1073/pnas.84.12.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitayaphan S., Omilianowski D., Toth M. M., Parks G. D., Rueckert R. R., Palmenberg A. C., Roos R. P. Relationship of Theiler's murine encephalomyelitis viruses to the cardiovirus genus of picornaviruses. Intervirology. 1986;26(3):140–148. doi: 10.1159/000149693. [DOI] [PubMed] [Google Scholar]
- Nitayaphan S., Toth M. M., Roos R. P. Neutralizing monoclonal antibodies to Theiler's murine encephalomyelitis viruses. J Virol. 1985 Feb;53(2):651–657. doi: 10.1128/jvi.53.2.651-657.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Stein S., Fu J. L., Stillman L., Klaman L., Roos R. P. Molecular cloning and sequence determination of DA strain of Theiler's murine encephalomyelitis viruses. Virology. 1988 May;164(1):245–255. doi: 10.1016/0042-6822(88)90642-3. [DOI] [PubMed] [Google Scholar]
- Ozden S., Aubert C., Brahic M. Expression of Theiler's murine encephalomyelitis virus protease 3C and polymerase 3D in Escherichia coli and characterization of monospecific sera. Virology. 1988 Aug;165(2):596–600. doi: 10.1016/0042-6822(88)90604-6. [DOI] [PubMed] [Google Scholar]
- Parks G. D., Duke G. M., Palmenberg A. C. Encephalomyocarditis virus 3C protease: efficient cell-free expression from clones which link viral 5' noncoding sequences to the P3 region. J Virol. 1986 Nov;60(2):376–384. doi: 10.1128/jvi.60.2.376-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear D. C., Borkowski J., Calenoff M., Oh C. K., Ostrowski B., Lipton H. L. Insights into Theiler's virus neurovirulence based on a genomic comparison of the neurovirulent GDVII and less virulent BeAn strains. Virology. 1988 Jul;165(1):1–12. doi: 10.1016/0042-6822(88)90652-6. [DOI] [PubMed] [Google Scholar]
- Pevear D. C., Calenoff M., Rozhon E., Lipton H. L. Analysis of the complete nucleotide sequence of the picornavirus Theiler's murine encephalomyelitis virus indicates that it is closely related to cardioviruses. J Virol. 1987 May;61(5):1507–1516. doi: 10.1128/jvi.61.5.1507-1516.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos R. P., Stein S., Ohara Y., Fu J. L., Semler B. L. Infectious cDNA clones of the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1989 Dec;63(12):5492–5496. doi: 10.1128/jvi.63.12.5492-5496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangar D. V., Newton S. E., Rowlands D. J., Clarke B. E. All foot and mouth disease virus serotypes initiate protein synthesis at two separate AUGs. Nucleic Acids Res. 1987 Apr 24;15(8):3305–3315. doi: 10.1093/nar/15.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Hanecak R., Dorner L. F., Anderson C. W., Wimmer E. Poliovirus RNA synthesis in vitro: structural elements and antibody inhibition. Virology. 1983 Apr 30;126(2):624–635. doi: 10.1016/s0042-6822(83)80018-x. [DOI] [PubMed] [Google Scholar]
- Strebel K., Beck E. A second protease of foot-and-mouth disease virus. J Virol. 1986 Jun;58(3):893–899. doi: 10.1128/jvi.58.3.893-899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Vakharia V. N., Devaney M. A., Moore D. M., Dunn J. J., Grubman M. J. Proteolytic processing of foot-and-mouth disease virus polyproteins expressed in a cell-free system from clone-derived transcripts. J Virol. 1987 Oct;61(10):3199–3207. doi: 10.1128/jvi.61.10.3199-3207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. In vitro molecular genetics as a tool for determining the differential cleavage specificities of the poliovirus 3C proteinase. Nucleic Acids Res. 1987 Mar 11;15(5):2069–2088. doi: 10.1093/nar/15.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. Processing determinants required for in vitro cleavage of the poliovirus P1 precursor to capsid proteins. J Virol. 1987 Oct;61(10):3181–3189. doi: 10.1128/jvi.61.10.3181-3189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]