Abstract
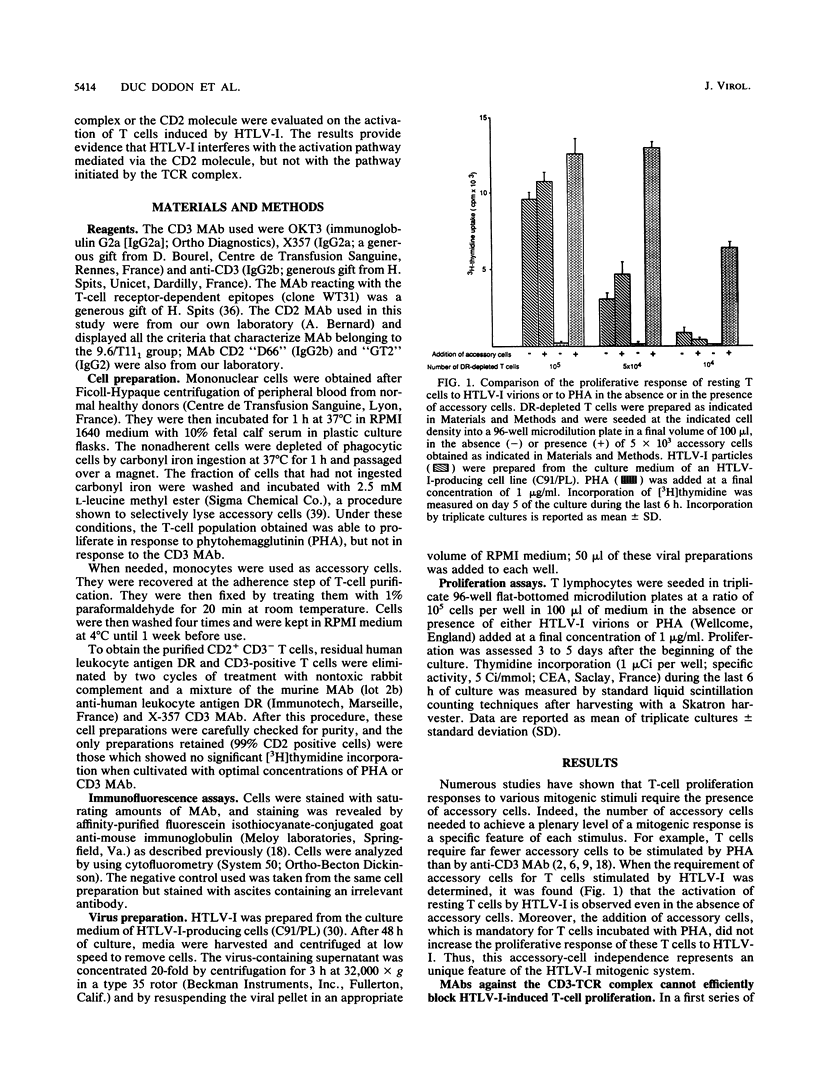
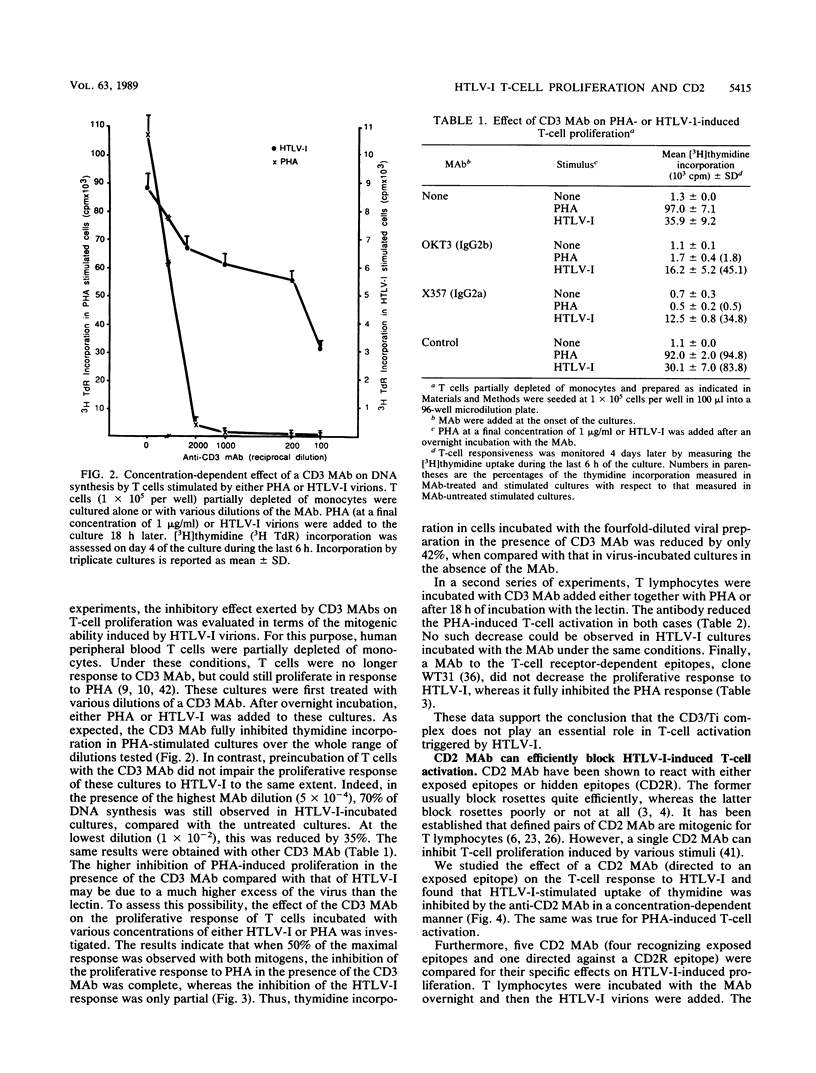
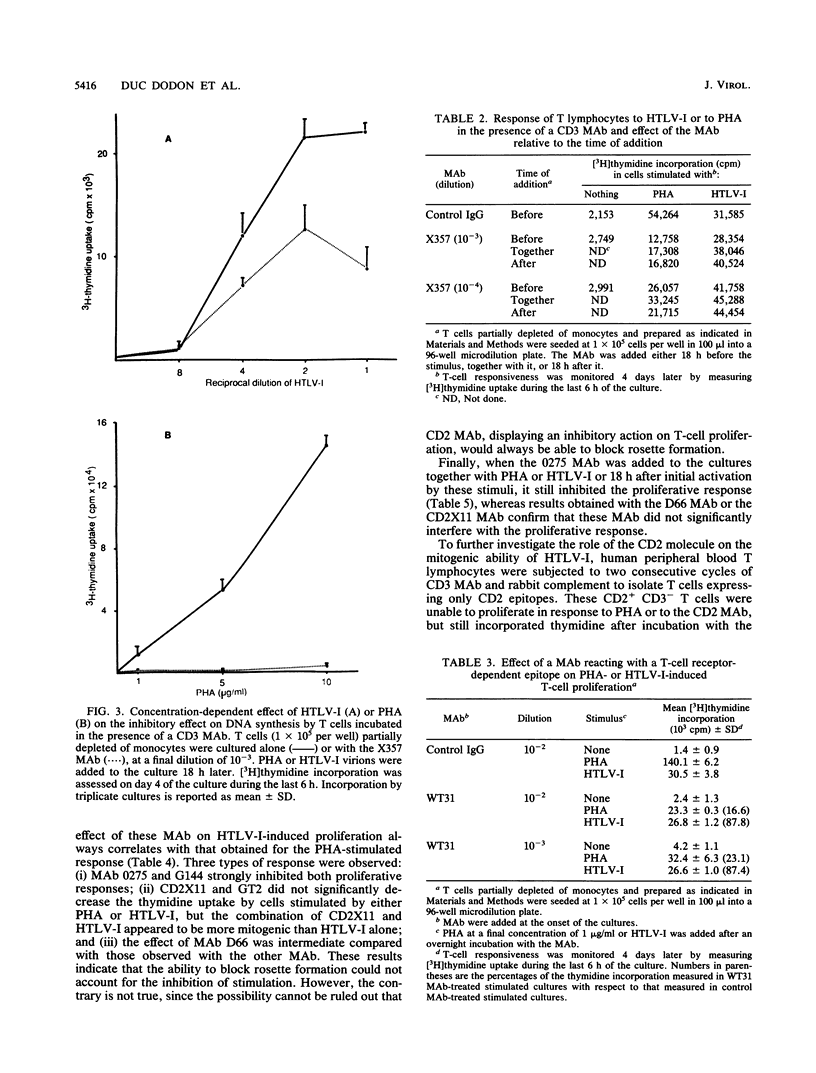
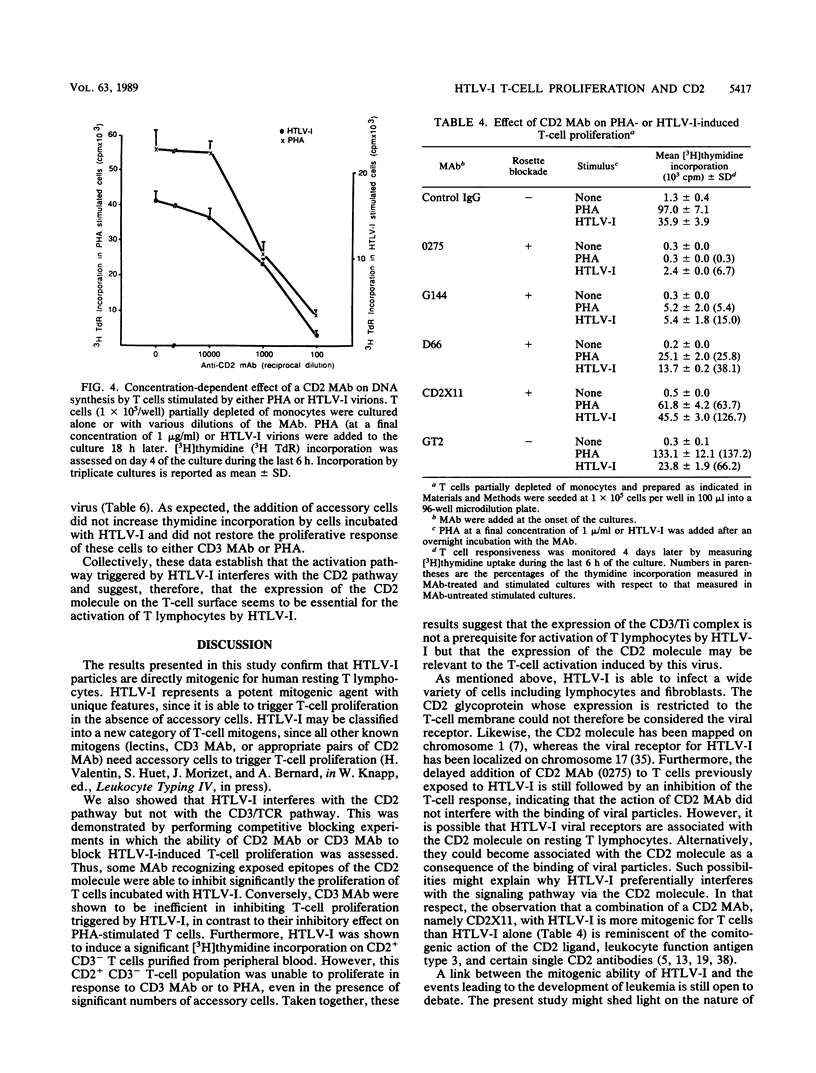
Human T-cell leukemia virus type I (HTLV-I) is etiologically associated with adult T-cell leukemia, an aggressive lymphoproliferative disorder, and with chronic neurological diseases. In vitro it can infect several types of cells but transforms only human T lymphocytes. We have previously shown that HTLV-I viral particles, even when noninfectious, were able to activate human resting T lymphocytes, suggesting that this activation step may be important in the initiation of the lymphoproliferative process. In the present study, we first demonstrate that in contrast to other mitogenic stimuli, HTLV-I has the unique property to activate human resting T cells in the absence of accessory cells. We then investigate the relationship between HTLV-I-induced T-cell activation and the classical well-known pathways of activation, namely, the CD3/TCR and CD2 pathways. Competitive blocking experiments were performed in which the effects of monoclonal antibodies (MAb) to the CD3/TCR complex or to the CD2 molecule were evaluated on the HTLV-I activation of T cells and compared with that obtained on phytohemagglutinin (PHA)-stimulated cells. It was found that anti-CD3 or -TCR MAb strongly suppress the proliferative response of T cells to PHA, but are significantly less efficient in inhibiting the activation initiated by HTLV-I. By contrast, MAb recognizing specific epitopes of the CD2 molecule inhibit the proliferative response of T cells to PHA or to HTLV-I to the same extent. The results provide evidence that HTLV-I virions interfere mainly with activation via CD2 but not via the CD3/TCR complex. Considering the earlier expression of the CD2 molecule on human T-cell precursors, these observations might be relevant to the characterization of the differentiation stage at which viral infection could interfere with the development and the maturation of T lymphocytes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988 Sep 23;241(4873):1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- Bekoff M., Kubo R., Grey H. M. Activation requirements for normal T cells: accessory cell-dependent and -independent stimulation by anti-receptor antibodies. J Immunol. 1986 Sep 1;137(5):1411–1419. [PubMed] [Google Scholar]
- Bernard A., Gelin C., Raynal B., Pham D., Gosse C., Boumsell L. Phenomenon of human T cells rosetting with sheep erythrocytes analyzed with monoclonal antibodies. "Modulation" of a partially hidden epitope determining the conditions of interaction between T cells and erythrocytes. J Exp Med. 1982 May 1;155(5):1317–1333. doi: 10.1084/jem.155.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt L. K., Goldsmith M. A., Dustin M., Olive D., Springer T. A., Weiss A. The CD2 ligand LFA-3 activates T cells but depends on the expression and function of the antigen receptor. J Immunol. 1988 Sep 15;141(6):1904–1911. [PubMed] [Google Scholar]
- Brottier P., Boumsell L., Gelin C., Bernard A. T cell activation via CD2 [T, gp50] molecules: accessory cells are required to trigger T cell activation via CD2-D66 plus CD2-9.6/T11(1) epitopes. J Immunol. 1985 Sep;135(3):1624–1631. [PubMed] [Google Scholar]
- Clayton L. K., Ramachandran H., Pravtcheva D., Chen Y. F., Diamond D. J., Ruddle F. H., Reinherz E. L. The gene for T11 (CD2) maps to chromosome 1 in humans and to chromosome 3 in mice. J Immunol. 1988 May 15;140(10):3617–3621. [PubMed] [Google Scholar]
- Copeland T. D., Tsai W. P., Kim Y. D., Oroszlan S. Envelope proteins of human T cell leukemia virus type I: characterization by antisera to synthetic peptides and identification of a natural epitope. J Immunol. 1986 Nov 1;137(9):2945–2951. [PubMed] [Google Scholar]
- Davis L., Lipsky P. E. Signals involved in T cell activation. II. Distinct roles of intact accessory cells, phorbol esters, and interleukin 1 in activation and cell cycle progression of resting T lymphocytes. J Immunol. 1986 May 15;136(10):3588–3596. [PubMed] [Google Scholar]
- Davis L., Vida R., Lipsky P. E. Regulation of human T lymphocyte mitogenesis by antibodies to CD3. J Immunol. 1986 Dec 15;137(12):3758–3767. [PubMed] [Google Scholar]
- Duc Dodon M., Gazzolo L. Loss of interleukin-2 requirement for the generation of T colonies defines an early event of human T-lymphotropic virus type I infection. Blood. 1987 Jan;69(1):12–17. [PubMed] [Google Scholar]
- Dumontet C., Dodon M. D., Gazzolo L., Gerlier D. Human T-cell leukemia virus type I-induced proliferation of human thymocytes requires the presence of a comitogen. Cell Immunol. 1988 Apr 1;112(2):391–401. doi: 10.1016/0008-8749(88)90308-5. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Olive D., Springer T. A. Correlation of CD2 binding and functional properties of multimeric and monomeric lymphocyte function-associated antigen 3. J Exp Med. 1989 Feb 1;169(2):503–517. doi: 10.1084/jem.169.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Bensussan A., Daley J. F., Schlossman S. F., Reinherz E. L. Activation of human thymocytes via the 50KD T11 sheep erythrocyte binding protein induces the expression of interleukin 2 receptors on both T3+ and T3- populations. J Immunol. 1985 Jan;134(1):330–335. [PubMed] [Google Scholar]
- Gazzolo L., Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987 Apr 16;326(6114):714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Martin M. E., Kay H. H., Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988 Sep 1;168(3):1061–1080. doi: 10.1084/jem.168.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet S., Wakasugi H., Sterkers G., Gilmour J., Tursz T., Boumsell L., Bernard A. T cell activation via CD2 [T, gp50]: the role of accessory cells in activating resting T cells via CD2. J Immunol. 1986 Sep 1;137(5):1420–1428. [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Homma T., McLane M. F., Tachibana N., Essex M. Human T-cell leukemia virus-associated membrane antigens: identity of the major antigens recognized after virus infection. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3856–3860. doi: 10.1073/pnas.81.12.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Trepel J., Cossman J., Mitsuya H., Broder S. Human monoclonal antibody directed against an envelope glycoprotein of human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2672–2676. doi: 10.1073/pnas.83.8.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Malefijt R. D., Heike T., Fujisawa J., Takebe Y., Nishida J., Shlomai J., Yokota T., Yoshida M. Activation of T cell-derived lymphokine genes in T cells and fibroblasts: effects of human T cell leukemia virus type I p40x protein and bovine papilloma virus encoded E2 protein. Nucleic Acids Res. 1988 Jul 25;16(14A):6547–6566. doi: 10.1093/nar/16.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Sarngadharan M. G., Matthews T. J. Purification of envelope glycoproteins of human T cell lymphotropic virus type I (HTLV-I) by affinity chromatography. J Virol Methods. 1987 Dec;18(4):243–255. doi: 10.1016/0166-0934(87)90086-3. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. D., Chen I. S., Wachsman W. Infection with HTLV-I and HTLV-II: evolving concepts. Semin Hematol. 1988 Jul;25(3):230–246. [PubMed] [Google Scholar]
- Schneider J., Yamamoto N., Hinuma Y., Hunsmann G. Sera from adult T-cell leukemia patients react with envelope and core polypeptides of adult T-cell leukemia virus. Virology. 1984 Jan 15;132(1):1–11. doi: 10.1016/0042-6822(84)90086-2. [DOI] [PubMed] [Google Scholar]
- Seiki M., Inoue J., Takeda T., Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986 Mar;5(3):561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekevitz M., Feinberg M. B., Holbrook N., Wong-Staal F., Greene W. C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfelt M. A., Williams B. P., Clapham P. R., Solomon E., Goodfellow P. N., Weiss R. A. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988 Dec 16;242(4885):1557–1559. doi: 10.1126/science.3201246. [DOI] [PubMed] [Google Scholar]
- Spits H., Borst J., Tax W., Capel P. J., Terhorst C., de Vries J. E. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. J Immunol. 1985 Sep;135(3):1922–1928. [PubMed] [Google Scholar]
- Sugamura K., Fujii M., Ueda S., Hinuma Y. Identification of a glycoprotein, gp21, of adult T cell leukemia virus by monoclonal antibody. J Immunol. 1984 Jun;132(6):3180–3184. [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Tiefenthaler G., Hünig T., Dustin M. L., Springer T. A., Meuer S. C. Purified lymphocyte function-associated antigen-3 and T11 target structure are active in CD2-mediated T cell stimulation. Eur J Immunol. 1987 Dec;17(12):1847–1850. doi: 10.1002/eji.1830171227. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Van Wauwe J. P., Goossens J., Van Nyen G. Inhibition of lymphocyte proliferation by monoclonal antibody directed against the T3 antigen on human T cells. Cell Immunol. 1984 Jul;86(2):525–534. doi: 10.1016/0008-8749(84)90408-8. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J., Goossens J., Decock W., Kung P., Goldstein G. Suppression of human T-cell mitogenesis and E-rosette formation by the monoclonal antibody OKT11A. Immunology. 1981 Dec;44(4):865–871. [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J. B. Cell surface molecules and early events involved in human T lymphocyte activation. Adv Immunol. 1987;41:1–38. doi: 10.1016/s0065-2776(08)60029-2. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack J. A., Cann A. J., Lugo J. P., Chen I. S. HIV-1 production from infected peripheral blood T cells after HTLV-I induced mitogenic stimulation. Science. 1988 May 20;240(4855):1026–1029. doi: 10.1126/science.2835813. [DOI] [PubMed] [Google Scholar]



