Abstract
Glutamate transporters in the central nervous system are expressed in both neurons and glia, they mediate high affinity, electrogenic uptake of glutamate, and they are associated with an anion conductance that is stoichiometrically uncoupled from glutamate flux. Although a complete cycle of transport may require 50–100 ms, previous studies suggest that transporters can alter synaptic currents on a much faster time scale. We find that application of l-glutamate to outside-out patches from cerebellar Bergmann glia activates anion-potentiated glutamate transporter currents that activate in <1 ms, suggesting an efficient mechanism for the capture of extrasynaptic glutamate. Stimulation in the granule cell layer in cerebellar slices elicits all or none α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor and glutamate transporter currents in Bergmann glia that have a rapid onset, suggesting that glutamate released from climbing fiber terminals escapes synaptic clefts and reaches glial membranes shortly after release. Comparison of the concentration dependence of both α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor and glutamate transporter kinetics in patches with the time course of climbing fiber-evoked responses indicates that the glutamate transient at Bergmann glial membranes reaches a lower concentration than attained in the synaptic cleft and remains elevated in the extrasynaptic space for many milliseconds.
Termination of the actions of synaptically released glutamate requires uptake by high affinity glutamate transporters. These transporters are expressed by both neurons and glia and maintain low extracellular glutamate levels by coupling translocation to the electrochemical gradients for Na+, K+, and H+ (1). The importance of these transporters in restricting glutamate neurotoxicity is evidenced by the physiological, behavioral, and anatomical abnormalities that result when their expression is reduced (2) or eliminated (3). On a faster time scale, glutamate transporters appear to be important in limiting the duration of synaptic excitation at some synapses (3, 4–7) by rapidly lowering the concentration of glutamate in the synaptic cleft following exocytosis; however, transporter antagonists do not prolong excitatory postsynaptic currents at all synapses (4, 8, 9) suggesting that other factors that vary between synapses such as receptor kinetics, location and density of transporters, and diffusional barriers may also be important in shaping the glutamate transient in the cleft. Glutamate transporters located near release sites have also been shown to slow the activation of postsynaptic ionotropic receptors (10, 11) suggesting that glutamate may bind to transporters within a millisecond after release. Such rapid binding kinetics have recently been demonstrated for glutamate transporters expressed in Purkinje cells (12). However, the lack of subtype-selective antagonists has hampered assessment of the relative contribution of neuronal and glial transporters to the uptake of glutamate on this time scale.
In the cerebellum, Bergmann glial processes ensheath excitatory synapses on Purkinje cells (13, 14), express high levels of the glutamate transporter GLAST (15, 16), and accumulate radiolabeled glutamate (17); they are therefore ideally positioned to capture glutamate that escapes from the synaptic cleft. Synaptic activation of glutamate transporters in Bergmann glia has been recently demonstrated in cerebellar slices (18) and are similar to the glutamate transporter currents elicited in cultured glial cells following neuronal stimulation (5, 19). These synaptic transporter currents begin shortly after stimulation suggesting that glutamate reaches sites on glial membranes within a millisecond after exocytosis. This observation is consistent with estimates of the diffusion rate of glutamate (20) as well as the decay rate of the glutamate transient in the cleft (11, 21). However, the amount of glutamate that escapes the cleft and the time that it remains elevated in the extrasynaptic space are not known. We describe the intrinsic kinetics of glial transporters in outside-out patches from Bergmann glial cells and compare these to α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor and transporter currents activated through climbing fiber (CF) stimulation in cerebellar slices to estimate the time course of glutamate in the extrasynaptic space. Our results indicate that the glutamate concentration at glial membranes peaks at a level much lower than the 1–3 mM achieved in the synaptic cleft (11, 21) and persists in extrasynaptic regions for >10 ms following release.
MATERIALS AND METHODS
Whole cell recordings and outside-out patches were obtained from Bergmann glia in cerebellar slices (300 μm) prepared from postnatal day (P) 11-P15 rats. Bergmann glia were visualized using a 40× water-immersion objective on an upright microscope (Zeiss Axioskop) equipped with IR/DIC optics. Patch pipettes had resistances of 2–4 MΩ when filled with K gluconate. The bath solution contained 119 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgCl2, 1 mM NaH2PO4, 26.2 mM NaHCO3, and 11 mM glucose, saturated with 95% O2/5% CO2. Pipette solutions contained 130 mM K+ A−, 20 mM Hepes, 10 mM EGTA, and 1 mM MgCl2, pH 7.2. A− denotes NO3−, SCN−, gluconate or methanesulfonate. Isolated AMPA responses were recorded in patches with an internal solution composed of 100 mM Cs2SO4, 20 mM Hepes, 10 mM EGTA, 4 mM MgATP, and 0.3 mM GTP, pH 7.2. CFs were stimulated (30–140 μA, 100 μs) with a theta glass pipette filled with bath solution that was placed in the granule cell layer. Synaptic currents were filtered at 1–2 kHz and digitized at 10 kHz, and patch currents were filtered at 5 kHz and digitized at 30–50 kHz. Current-clamp responses were recorded with an Axoclamp-2A, while synaptic and patch currents were recorded with an Axopatch-200A. When recording synaptic currents, access resistance was monitored throughout the experiment and averaged 5.5 ± 2.3 MΩ; if it changed >20%, the experiment was discarded. Holding potentials have been corrected for junction potentials. For morphological identification, Bergmann glia were filled with 1.5 mM Cy5-EDA (Amersham Life Science) in KNO3 via the patch pipette and imaged on a confocal microscope (Odyssey XL, Noran Instruments, Middleton, WI). Rapid agonist applications to outside-out patches were performed as described (10) using an extracellular solution containing 135 mM NaCl, 5.4 mM KCl, 5 mM Hepes, 1.8 mM CaCl2, 1.3 mM MgCl2, at pH 7.2. Concentration-response was performed on individual patches by connecting a four-barrel miniature manifold (Warner Instruments, Hamden, CT) to one side of a theta pipette. Artifacts arising from voltage steps applied to the bimorph have been blanked. Values are given as mean ± SD. All experiments were performed at room temperature (22–24°C).
RESULTS
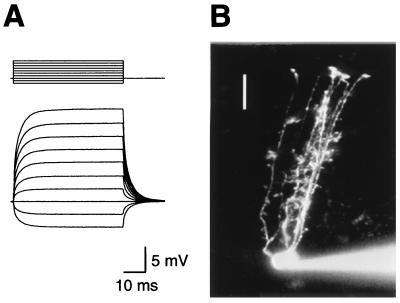
Bergmann glial cell bodies were initially identified in slices of rat cerebellum by their small size (≈10 μm in diameter) and their location in the Purkinje cell layer. Whole-cell recordings from these cells revealed their characteristic low input resistances (30 ± 21 MΩ, n = 24), high resting membrane potentials (−87 ± 6 mV, n = 24), and lack of voltage dependent currents (Fig. 1A). Biexponential fits to the passive membrane responses to current steps yielded time constants of: τfast = 0.43 ± 0.25 ms, 51 ± 12%; τslow = 2.4 ± 0.4 ms; n = 6). Cells exhibiting these electrophysiological properties were filled the dye, Cy5-EDA, revealing that they typically had five or more thin, apically projecting processes that were interrupted along their length by irregular protrusions before terminating at the pial surface with rounded expansions (Fig. 1B). These morphological features are characteristic of Bergmann glia (13). Rapid application of l-glutamate (10 mM) to outside-out patches from Bergmann glial cells activated both an AMPA receptor-mediated current that was blocked by 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo(F)quinoxaline (NBQX) and 1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3 benzodiazepine hydrochloride (GYKI-52466), and a current that was insensitive to these antagonists when the pipette contained SCN−, which is highly permeant through glial glutamate transporters (22) (Fig. 2A). In the absence of a permeant anion, the current remaining in AMPA receptor antagonists was very small (<5 pA; n = 7). Glutamate transporters, unlike ionotropic receptors, show a near absolute dependence on external Na+ (1). When external Na+ was replaced by Li+ (135 mM) the NBQX/GYKI-insensitive current was blocked (n = 5; Fig. 2B). These data suggest that both AMPA receptors and glutamate transporters are present in patches removed from the somata of Bergmann glial cells.
Figure 1.
Identification of a Bergmann glial cell. (A) Voltage responses of a Bergmann glia cell to 50 ms current steps (−200 pA to 700 pA) from a resting potential of −87 mV. Fitting the rising phases of these responses with two exponentials gave a fast time constant of 0.36 ms (47.3% amplitude) and a slower time constant of 3.3 ms. The time constants for the rise and decay phases were not significantly different (P > 0.05). This cell had an input resistance of 27 MΩ. (B) The cell was recorded with a patch pipette filled with 1.5 mM Cy5-EDA in KNO3 internal solution and simultaneously imaged on a confocal microscope. Image is a composite of 11 optical sections taken at 5 μm steps. Bar = 25 μm.
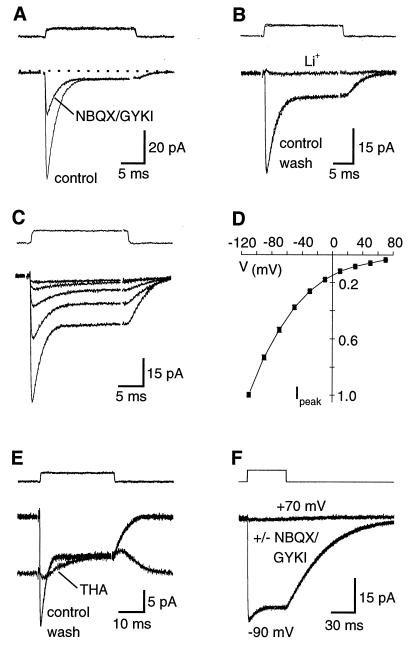
Figure 2.
Glutamate transporter currents and AMPA receptor currents can be evoked in outside-out patches from Bergmann glia. (A) l-glutamate (10 mM) activates a transient current that is blocked by NBQX and GYKI-52466 and a smaller biphasic current in patches using a KSCN internal solution. Vm = −90 mV. (B) Complete substitution of extracellular Li+ for Na+ blocks the transporter current evoked by 10 mM l-glutamate. Vm = −90 mV. (C and D) The current-voltage relationship of the transporter current is inwardly rectifying and does not reverse. Responses in C were recorded at membrane potentials of −10, −30, −50, −70, and −90 mV. In D, each point is an average of responses from six patches normalized to the peak response at −110 mV. (E) In the presence of NBQX (10 μM) and GYKI (25 μM), THA (300 μM) produces a steady-state inward current and occludes the response to a pulse of 10 mM l-glutamate. The outward current elicited by l-glutamate in the presence of THA probably reflects the replacement of THA with l-glutamate, which is likely to occur at these concentrations. Vm = −110 mV. (F) l-aspartate (2 mM) activates a transporter current but not an AMPA receptor current. The control responses and those recorded in NBQX (10 μM) and GYKI (25 μM) are superimposed. This result also indicates that at these concentrations neither NBQX nor GYKI alter transporter function. KSCN-based internal solutions were used in all recordings. The uppermost traces in each figure are the “open-tip” response obtained by solution exchanges after disrupting the membrane patch. In each case the concentrations listed were used, except in F, where ACSF was diluted with 50% dH2O to increase the size of the junction current. Traces are averages of 5–15 responses.
The current-to-voltage relation of l-glutamate evoked transporter currents rectified inwardly and did not reverse at positive potentials (n = 6; Figs. 2 C and D), as would be expected under these ionic conditions (22). Transporter currents in patches were blocked by the broad spectrum substrate/antagonist d,l-threo-β-hydroxyaspartic acod (THA) (300 μM; 92 ± 4% inhibition; n = 11; Fig. 2E), but were not substantially affected by DHK (300 μM; 9 ± 3% inhibition; n = 6), a selective inhibitor for the GLT-1 transporter (23). These data are consistent with the predominant expression of GLAST in Bergmann glia at this age (24). l-glutamate reduced the standing inward current produced by THA (Fig. 2E). This may reflect the replacement of THA with l-glutamate as THA unbinds or is transported, causing the larger steady-state response produced by THA to decay to the steady-state level produced by l-glutamate. l-aspartate (2 mM) also evoked a current in patches that was insensitive to NBQX/GYKI and remained inward at potentials up to 70 mV (n = 6; Fig. 2F), consistent with the selective activation of glutamate transporters (25) and not AMPA receptors (26).
Transporter currents decayed to steady-state levels in the continued presence of l-glutamate. The explanation for the transient transporter current is not known, in part, because it comprises both the charge movement coupled to glutamate translocation and the uncoupled anion current. The decay to a steady-state level may reflect desynchronization of the transporters (27) as they reach nonconducting states in the transport cycle. l-aspartate evoked transporter currents with slower kinetics and larger steady-state components than those elicited by l-glutamate, possibly reflecting the higher apparent affinity and slower transport rate of l-aspartate (23, 25).
A recent study demonstrated that glutamate released following parallel fiber stimulation in cerebellar slices activates both AMPA receptors and transporters in glial membranes (18). In addition to parallel fibers, Purkinje cells receive powerful excitatory input from single CFs that form contacts with 1,000 or more spines on individual Purkinje cells (13). These terminals are also ensheathed by Bergmann glial processes (13, 14), suggesting that glutamate released from CF synapses might also reach receptors and transporters in Bergmann glial membranes. Focal stimulation in the granule cell layer elicited all-or-none, inward currents in Bergmann glia voltage-clamped at their resting potential, consistent with activation of a single CF (28)(Fig. 3 A and B). Unlike parallel fiber responses in Bergmann glia (18), CF currents were not contaminated by the detection of the extracellular field response through the low resistance glial membrane, a consequence of the activation of many parallel fibers. CF-evoked currents had two kinetically distinct components that could be separated pharmacologically. Addition of antagonists of ionotropic receptors, NBQX, R(-)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (D-CPP), and SR-95531, inhibited a component that had rapid kinetics (n = 24; Fig. 3C). The time course of this component, determined by subtraction of the remaining slower current (Fig. 3D), had a 20–80% rise time of 1.0 ± 0.5 ms and a half-decay time of 4.2 ± 2.0 ms (n = 24). This fast response was also blocked by NBQX alone (10 μM; n = 3) indicating that it was mediated by AMPA receptors. The component remaining in NBQX had a 20–80% rise time of 1.9 ± 0.7 ms, and a half-decay of 17.3 ± 4.2 ms (n = 24). This slow response was blocked by Cd2+ (30 μM, n = 5; Fig. 3E), and was inhibited by 93 ± 8% by THA (300 μM, n = 15; Fig. 3C), suggesting that it reflects the activation of glutamate transporters by glutamate released following Ca2+-dependent exocytosis at CF terminals. Consistent with the results obtained from patch experiments, dihydrokainate (300 μM) inhibited this current by only 3.8 ± 4.9% (n = 6; Fig. 3E) indicating that it is mediated primarily by GLAST, the predominant glutamate transporter expressed in Bergmann glia (15, 16).
Figure 3.
CF stimulation elicits both glutamate transporter currents and AMPA receptor currents in Bergmann glia. (A) Bergmann glia response to CF stimulation is all-or-none and blocked by a combination of NBQX (10 μM), D-CPP (5 μM), SR-95531 (5 μM), and THA (300 μM). Each point represents the average of three consecutive responses. Holding potential was −90 mV. At the first asterisk the stimulation intensity was decreased to 10 μA, below the threshold for the response. The stimulation intensity was then increased in steps of 20 μA, with three responses recorded at each intensity. The response returned at 70 μA and did not increase in amplitude when the intensity was increased further (up to 130 μA). A stimulation intensity of 110 μA was maintained during the rest of the experiment except at the second asterisk where the threshold was again tested. (B) Averaged Bergmann glial responses to stimuli of different intensities. (C) The rapid component of the current is blocked by NBQX (10 μM) and the remaining slow component is blocked by THA (300 μM). (D) Comparison of the kinetics of the AMPA receptor and transporter currents evoked by CF stimulation. The AMPA receptor current was obtained by subtracting the response in the presence of NBQX from the control response. (E) CdCl2 (30 μM), but not dihydrokainate (300 μM), blocked the NBQX-insensitive current. All traces are averages of 3–5 consecutive responses and were recorded from the same Bergmann glial cell. KNO3-based internal solution.
In recordings where NO3− was the primary anion in the pipette solution (e.g., Fig. 3), which is also highly permeant through GLAST (22), the amplitude of the CF-evoked transporter current was 44 ± 13% of the control response in the absence of ionotropic receptor antagonists (n = 17), whereas when the impermeant anion gluconate was the primary anion, the transporter current was 30 ± 16% of control (n = 7; P = 0.017). These results are consistent with a potentiation of the transporter current by an associated anion conductance. However, the time course of the synaptic transporter currents were not different in cells with the two internal solutions (20–80% rise times: 1.8 ± 0.5 ms vs. 2.2 ± 0.9 ms; half-decay times: 17.2 ± 4.4 ms vs. 17.7 ± 4.0 ms; for NO3− and gluconate-filled cells, respectively). It was not possible to use a SCN−-based internal solution to record CF responses, because a large SCN− leakage current was induced upon breaking-in and cells tended to visibly shrink over time, confounding recordings of evoked responses.
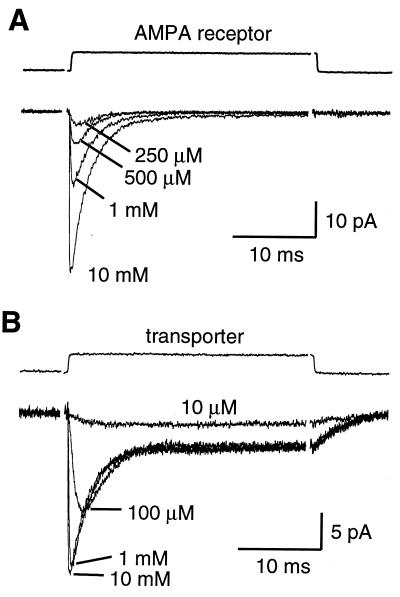
To estimate the glutamate concentration profile at Bergmann glial membranes following CF stimulation, the concentration dependence of the kinetics of AMPA receptor and transporter currents in patches was compared with that of the synaptically activated currents. The rise (20–80%) and half-decay times of AMPA receptor currents in patches to steps of l-glutamate were 130 ± 40 μs and 1.6 ± 0.2 ms at 10 mM, 260 ± 120 μs and 1.7 ± 0.1 ms at 1 mM, 320 ± 60 μs and 2.0 ± 0.5 ms at 500 μM, and 600 ± 190 μs and 2.4 ± 0.6 ms at 250 μM (n = 5; Fig. 4A). If the AMPA receptors expressed in Bergmann glia were to sense an instantaneous rise of glutamate following CF stimulation, the rise and half-decay times of the synaptic current (1.0 and 4.2 ms) would be consistent with an average peak concentration of <250 μM.
Figure 4.
Concentration-dependence of the kinetics of glutamate transporter and AMPA receptor currents in patches from Bergmann glial cells. (A) Currents evoked in an outside-out patch by 30 ms applications of l-glutamate at 250 μM, 500 μM, 1 mM and 10 mM. Cs2SO4-based internal solution. (B) Currents evoked in an outside-out patch in the presence of NBQX (10 μM) and GYKI (25 μM) by applications of l-glutamate at 10 μM, 100 μM, 1 mM and 10 mM. KSCN internal solution. Traces are averages of 15–50 responses. Vm = −90 mV.
The rise time (20–80%) of transporter currents in patches to steps of l-glutamate were also very fast relative to the synaptic transporter currents, except at 10 μM: 206 ± 39 μs at 10 mM, 287 ± 30 μs at 1 mM, 780 ± 75 μs at 100 μM, and 3.12 ± 0.68 ms at 10 μM (n = 6; Fig. 4B). As the rise time of the synaptic transporter current was 1.9 ms, the patch data imply that glutamate reaches a peak concentration of 10 to 100 μM at these extrasynaptic sites. In addition, the decay time of synaptic transporter currents (17.3 ms) was slow relative to the decay of patch responses during steps of glutamate (half-decays of 2.8 and 3.9 ms for steps of 10 mM and 100 μM l-glutamate, respectively) as well as at the end of glutamate applications (1 ms pulse, 1.7 ± 0.2 ms, n = 3; 30 ms pulse, 2.7 ± 0.3 ms, n = 7). These data suggest that glutamate can remain elevated for >10 ms at Bergmann glial membranes following exocytosis.
DISCUSSION
Our results demonstrate that glutamate transporters expressed in Bergmann glial cells have rapid kinetics and are capable of binding glutamate on a submillisecond time scale, similar to the binding rates estimated for ionotropic glutamate receptors (29). This rapid binding of glutamate may provide an efficient mechanism for capturing glutamate that escapes the synaptic cleft, reducing the time that glutamate is elevated in the extracellular space following release. The rapid charge movement described here contrasts with the time required to complete a transport cycle, estimated at 70 ms for the glial glutamate transporter GLT-1 (30). The exact steps in the transport cycle that permit the flow of anions are not yet known, however the rapid onset of the anionic current and the demonstration that mutant transporters that are deficient in K+ binding have an anion conductance that is indistinguishable from wild type (31), suggest that it reflects early steps in the transport cycle and may parallel the transit of glutamate.
In hippocampal cultures the concentration of glutamate in the synaptic cleft following release decays from 1–3 mM to tens of micromolar within several ms after release, due to rapid diffusion out of the cleft and buffering by transporters (10, 11, 21). Stimulation of single CFs elicited both AMPA receptor and glutamate transporter currents in Bergmann glia that had rapid onsets, suggesting that substantial glutamate must diffuse out of the synaptic cleft shortly after release. This is consistent with the binding and perhaps transport of only 22% of the glutamate released by postsynaptic transporters (12), though it is not known how much is taken up into CF terminals. Comparison of the time course of this synaptic current and the rapid kinetics of AMPA receptors measured in patches suggests that the glutamate transient at the Bergmann glial membranes nearby synapses peaks within a millisecond, though at a significantly lower concentration than is thought to occur in the synaptic cleft (11, 21). The slow decay of the synaptic transporter current indicates that clearance of glutamate from extrasynaptic sites requires >10 ms. The differences in the clearance times of synaptic and extrasynaptic spaces could be explained by a clustering of transporters near release sites, although clearance at CF synaptic clefts may be slower than those in hippocampal cultures (32, 12). These estimates assume that the receptors and transporters are not altered by patch excision and that those expressed at the soma are representative of those detecting glutamate released from synapses.
The response to brief applications of a high concentration of l-glutamate to ionotropic receptors in patches often mimic the time course of miniature AMPA excitatory postsynaptic currents, demonstrating that the kinetics of the receptors are the primary determinants of the response time course in neurons (33). In contrast, the kinetics of AMPA receptors and transporters in patches from Bergmann glia were faster than responses elicited through CF stimulation. A number of factors may account for the slower time course of these synaptic responses including electrotonic filtering, release asynchrony, and diffusion of transmitter to distant sites. The degree of slowing of the synaptic responses by filtering will depend on the location of the receptors and transporters, the active and passive properties of Bergmann glial membranes, the location of conductances, and the internal resistance of the processes, none of which are known. However, the membrane time constant of Bergmann glia is fast (see Fig. 1A) suggesting that charging of the membrane should occur rapidly, minimizing the effects of electrotonic filtering (34). If AMPA receptors and transporters have similar locations, the degree of slowing of the transporter current should be less because of its slower time course.
Release asynchrony may slow the time course of the synaptic response as exocytosis does not occur at all synapses simultaneously following Ca2+ influx into presynaptic terminals. Although release asynchrony has not been measured at CF inputs, at auditory nerve inputs to the anteroventral cochlear nucleus that have similarly high release probabilities, release is complete in 1–2 ms (35), too fast to account for the slow time course of the synaptic responses described here. In addition, CF-evoked AMPA responses in Bergmann glia had a faster time course than the evoked transporter current, even though the degree of slowing due to asynchrony should be the same, suggesting that the slower time course of the transporter current does not result from release asynchrony.
Unlike postsynaptic ionotropic receptors that are clustered opposite release sites, the receptors and transporters expressed by Bergmann glia may be spread over several micrometers of membrane surrounding synaptic clefts (36, 37). This spread may slow the rise time of the responses, as activation would be staggered in time as glutamate diffuses to distant receptors. In addition, receptors and transporters located further away from the synaptic clefts would experience a lower concentration of transmitter, slowing activation due to the concentration dependence of binding. As the decay of the AMPA receptor and transporter responses in patches were slower in response to lower concentrations of l-glutamate, this dilution of glutamate could also contribute to the slow decay of synaptic responses. The higher apparent affinity of the glutamate transporters, estimated from the dose-response relationship (EC50 (transporter) = 58 μM; n = 8 vs. EC50 (AMPA receptor) = 1.15 mM; n = 5; see Fig. 4), suggests that they will be activated further away from release sites where the concentration of glutamate would be too low for significant AMPA receptor activation.
A number of studies have suggested that aspartate is released from CFs, although the >10 fold greater affinity of vesicular transporters for glutamate has cast doubt on this hypothesis (38). The data presented here suggest that aspartate cannot be the sole CF transmitter, as substantial glutamate must be released to account for the activation of AMPA receptors (26). However, these results do not exclude the possibility that some aspartate is released along with glutamate. The kinetics of transporter currents in patches elicited by l-aspartate were dramatically slower than those elicited by l-glutamate (compare Fig. 2 E and F), possibly reflecting the higher affinity of this agonist for these transporters (Km = 6.5 ± 3 μM, l-aspartate; Km = 12 ± 4 μM, l-glutamate)(25). If aspartate were released with glutamate the time course of the synaptically activated transport current would be slower, with the degree of slowing dependent on the relative proportion of aspartate to glutamate released.
Although micromolar levels of ambient glutamate have been measured in the extracellular space using microdialysis techniques (2) and theoretical predictions based on the stoichiometry of transport suggest that transporters may be able to lower extracellular glutamate to submicromolar levels at equilibrium (39), the data presented here suggest that the concentration of glutamate outside the synaptic cleft is not static but fluctuates in the vicinity of synapses where release has occurred. The fact that glutamate escapes the cleft and remains elevated extrasynaptically for several ms suggests that glial transporters may determine the degree to which receptors located outside the cleft are activated following each release event.
Acknowledgments
We thank J. S. Diamond, M. P. Kavanaugh, T. S. Otis, and J. I. Wadiche for helpful discussions, and J. T. Williams for assistance with confocal microscopy. This work was supported by the National Institutes of Health.
ABBREVIATIONS
- CF
climbing fiber
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline
- THA
d,l-threo-β-hydroxyaspartic acid
- GYKI-52466
1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride
References
- 1.Robinson M B, Dowd L A. Adv Pharmacol. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- 2.Rothstein J D, Dykes-Hoberg M, Pardo C A, Bristol L A, Jin L, Kuncl R W, Kanai Y, Hediger M A, Wang Y, Schielke J P, Welty D F. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 4.Sarantis M, Ballerini L, Miller B, Silver R A, Edwards M, Attwell D. Neuron. 1993;11:541–549. doi: 10.1016/0896-6273(93)90158-n. [DOI] [PubMed] [Google Scholar]
- 5.Mennerick S, Zorumski C F. Nature (London) 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 6.Otis T, Wu Y-C, Trussell L O. J Neurosci. 1996;16:1634–1644. doi: 10.1523/JNEUROSCI.16-05-01634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi M, Sarantis M, Attwell D. J Physiol (London) 1996;497:523–530. doi: 10.1113/jphysiol.1996.sp021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hestrin S, Sah P, Nicoll R A. Neuron. 1990;5:247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- 9.Isaacson J S, Nicoll R A. J Neurophysiol. 1993;70:2187–2191. doi: 10.1152/jn.1993.70.5.2187. [DOI] [PubMed] [Google Scholar]
- 10.Tong G, Jahr C E. Neuron. 1994;13:1195–1203. doi: 10.1016/0896-6273(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 11.Diamond J S, Jahr C E. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otis T S, Kavanaugh M P, Jahr C E. Science. 1997;277:1515–1518. doi: 10.1126/science.277.5331.1515. [DOI] [PubMed] [Google Scholar]
- 13.Palay S L, Chan-Palay V. Cerebellar Cortex, Cytology and Organization. New York: Springer; 1974. [Google Scholar]
- 14.Spacek J. Anat Embryol. 1985;171:245–252. doi: 10.1007/BF00341419. [DOI] [PubMed] [Google Scholar]
- 15.Rothstein J D, Martin L, Levey A I, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl R W. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 16.Lehre K P, Levy L M, Ottersen O P, Storm-Mathisen J, Danbolt N C. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Barry J, Langley G, Vincendon G, Gombos G. Neuroscience. 1982;7:1289–1297. doi: 10.1016/0306-4522(82)91134-4. [DOI] [PubMed] [Google Scholar]
- 18.Clark B A, Barbour B. J Physiol (London) 1997;502:335–350. doi: 10.1111/j.1469-7793.1997.335bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linden D J. Neuron. 1997;18:983–994. doi: 10.1016/s0896-6273(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 20.Wahl L M, Pouzat C, Stratford K J. J Neurophysiol. 1996;75:597–608. doi: 10.1152/jn.1996.75.2.597. [DOI] [PubMed] [Google Scholar]
- 21.Clements J D, Lester R A J, Tong G, Jahr C E, Westbrook G L. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 22.Wadiche J I, Amara S G, Kavanaugh M P. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 23.Arriza J L, Fairman W A, Wadiche J I, Murdoch G H, Kavanaugh M P, Amara S G. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata T, Watanabe M, Tanaka K, Wada K, Inoue Y. NeuroReport. 1996;7:705–709. doi: 10.1097/00001756-199602290-00006. [DOI] [PubMed] [Google Scholar]
- 25.Klockner U, Storck T, Conradt M, Stoffel W. J Neurosci. 1994;14:5759–5765. doi: 10.1523/JNEUROSCI.14-10-05759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patneau D K, Mayer M L. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruns D, Engert F, Lux H-D. Neuron. 1993;10:559–572. doi: 10.1016/0896-6273(93)90159-o. [DOI] [PubMed] [Google Scholar]
- 28.Ito M. The Cerebellum and Neuronal Control. New York: Raven; 1984. [Google Scholar]
- 29.Jonas P, Sakmann B. J Physiol (London) 1992;455:143–171. doi: 10.1113/jphysiol.1992.sp019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadiche J I, Arriza J L, Amara S G, Kavanaugh M P. Neuron. 1995;14:1019–1027. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 31.Kavanaugh M P, Bendahan A, Zerangue N, Zhang Y, Kanner B I. J Biol Chem. 1997;272:1703–1708. doi: 10.1074/jbc.272.3.1703. [DOI] [PubMed] [Google Scholar]
- 32.Barbour B, Keller B U, Llano I, Marty A. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 33.Jonas P, Spruston N. Curr Biol. 1994;4:366–372. doi: 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Trussell L O. J Neurophysiol. 1994;72:705–718. doi: 10.1152/jn.1994.72.2.705. [DOI] [PubMed] [Google Scholar]
- 35.Isaacson J S, Walmsley B. Neuron. 1995;15:1–20. doi: 10.1016/0896-6273(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 36.Baude A, Molnar E, Latawiec R A, McIlhinney J, Somogyi P. J Neurosci. 1994;14:2830–2843. doi: 10.1523/JNEUROSCI.14-05-02830.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudry F A, Lehre K, van Lookeren Campagne M, Ottersen O P, Danbolt N C, Storm-Mathisen J. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls D, Attwell D. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- 39.Zerangue N, Kavanaugh M P. Nature (London) 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]