Abstract
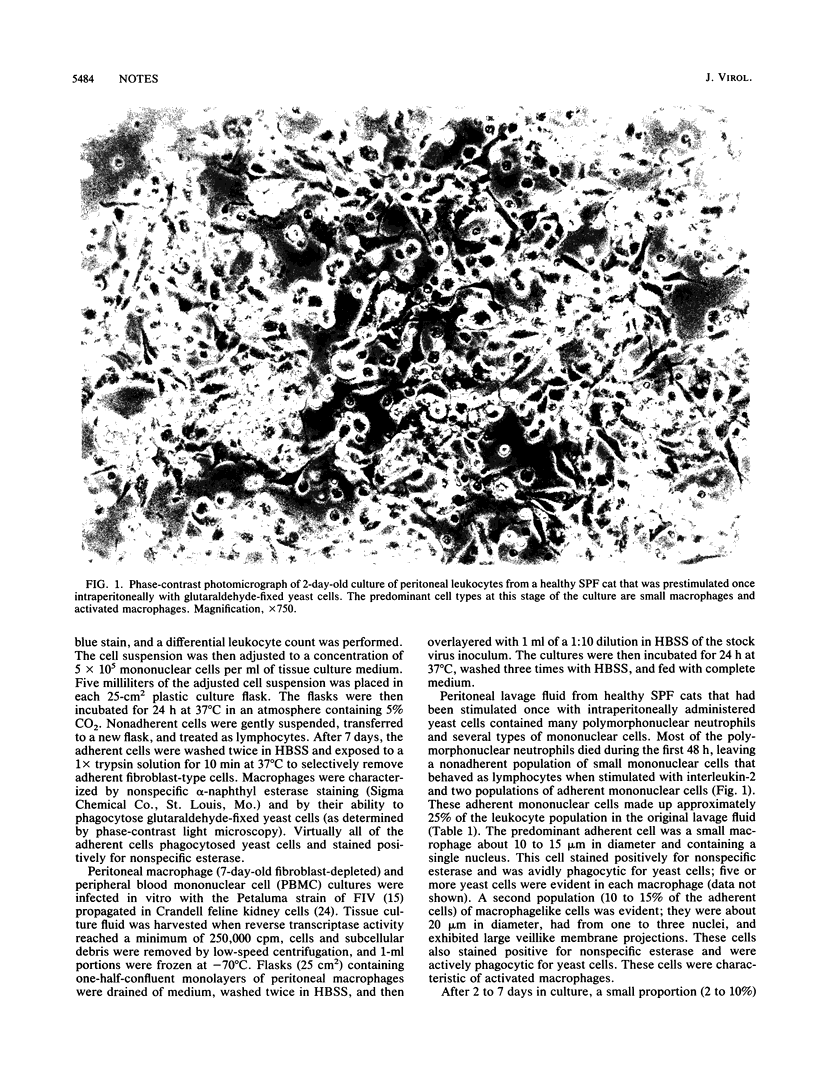
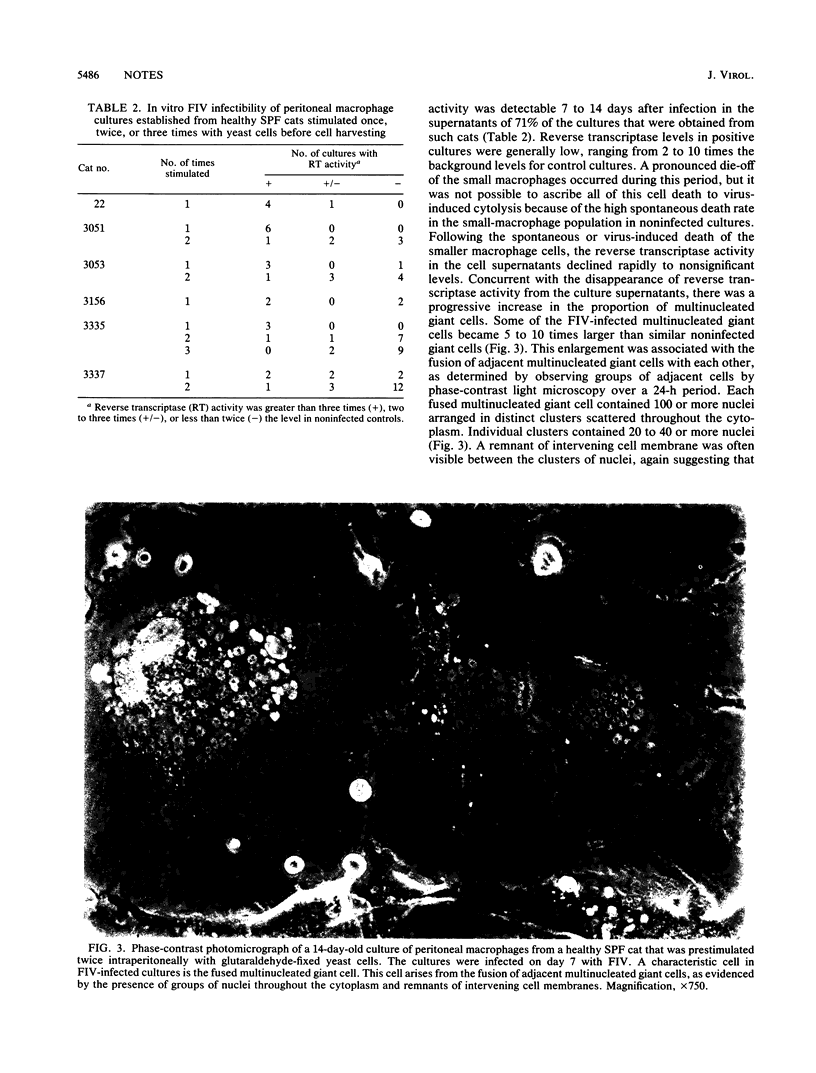
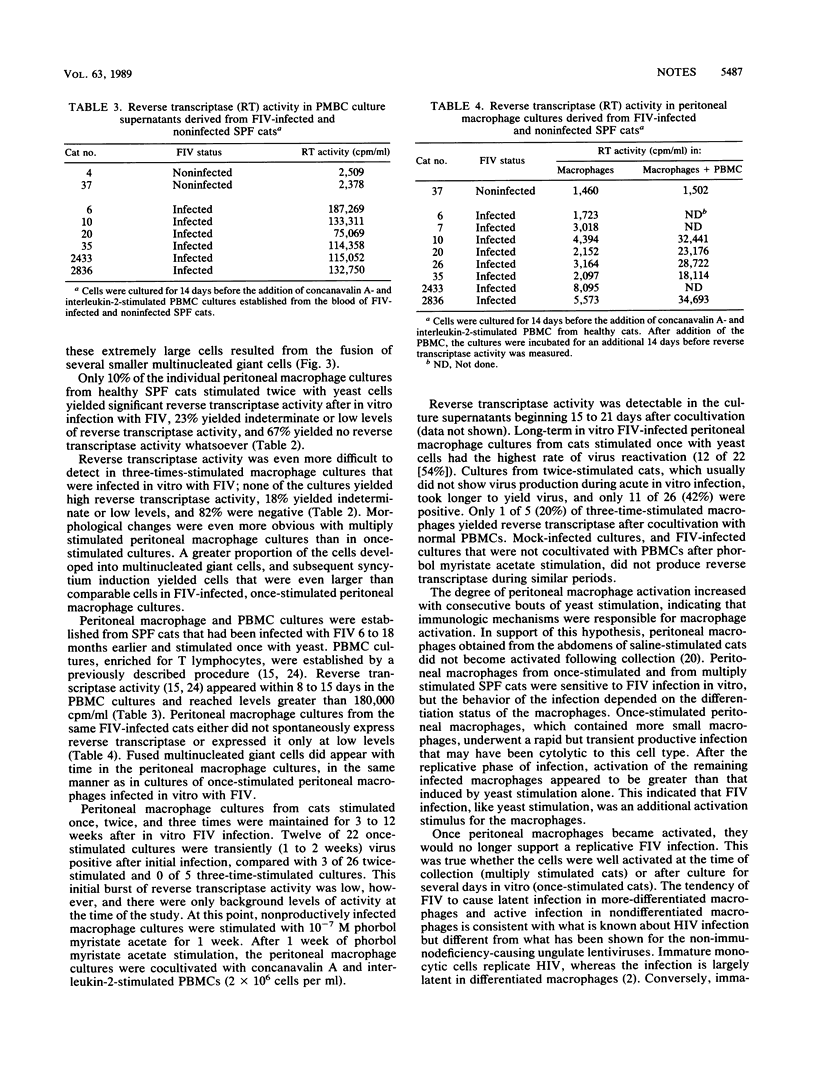
Macrophages were harvested from the peritoneal cavities of healthy specific-pathogen-free cats by saline lavage. Three days before collection, the peritoneal cavities were stimulated with glutaraldehyde-fixed Saccharomyces cerevisiae cells to induce greater numbers of macrophages and to begin the activation sequence. Peritoneal macrophages from cats stimulated once with yeast consisted mainly of small macrophages and a smaller number of larger activated macrophages. After several days in culture, many of the small macrophages became activated and a portion of the activated macrophages developed into multinucleated giant cells. Peritoneal cells from cats that were stimulated twice or three times with yeast at 3-week intervals consisted of a higher proportion of activated macrophages initially and produced more and larger multinuclear giant cells with time. Cultures of peritoneal cells stimulated once with yeast were easily infected in vitro with feline immunodeficiency virus (FIV) and produced a transient burst of reverse transcriptase activity. After the initial burst of virus replication, the infection became latent. Even more multinucleated giant cells appeared after infection, and many of these cells fused with each other. Replicating virus could be rescued from the latently infected macrophages after 2 to 3 weeks of phorbol myristate acetate stimulation and cocultivation with T-lymphocyte-enriched peripheral blood mononuclear cells. Multiply stimulated peritoneal cells, which contained a much higher proportion of activated macrophages, could also be infected in vitro with FIV. The infection usually became latent, however, without going through an initial replicative stage. Peritoneal cells from chronically FIV-infected specific-pathogen-free cats contained a higher proportion of activated macrophages and were latently infected with FIV from the outset.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature. 1989 May 4;339(6219):70–73. doi: 10.1038/339070a0. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Rojko J. L., Wilson P. L., Olsen R. G. Determinants of susceptibility and resistance to feline leukemia virus infection. I. Role of macrophages. J Natl Cancer Inst. 1981 Oct;67(4):889–898. doi: 10.1093/jnci/67.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Current concepts: immunology. Monocytes and macrophages. N Engl J Med. 1988 Mar 24;318(12):747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Le Tourneau A., Audouin J., Diebold J., Marche C., Tricottet V., Reynes M. LAV-like viral particles in lymph node germinal centers in patients with the persistent lymphadenopathy syndrome and the acquired immunodeficiency syndrome-related complex: an ultrastructural study of 30 cases. Hum Pathol. 1986 Oct;17(10):1047–1053. doi: 10.1016/s0046-8177(86)80089-2. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., O'Rourke K., Cheevers W. P. A review of antigenic variation by the equine infectious anemia virus. Contrib Microbiol Immunol. 1987;8:77–89. [PubMed] [Google Scholar]
- Meyenhofer M. F., Epstein L. G., Cho E. S., Sharer L. R. Ultrastructural morphology and intracellular production of human immunodeficiency virus (HIV) in brain. J Neuropathol Exp Neurol. 1987 Jul;46(4):474–484. doi: 10.1097/00005072-198707000-00006. [DOI] [PubMed] [Google Scholar]
- Nabel G., Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987 Apr 16;326(6114):711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- Narayan O., Clements J., Kennedy-Stoskopf S., Sheffer D., Royal W. Mechanisms of escape of visna lentiviruses from immunological control. Contrib Microbiol Immunol. 1987;8:60–76. [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauza C. D. HIV persistence in monocytes leads to pathogenesis and AIDS. Cell Immunol. 1988 Apr 1;112(2):414–424. doi: 10.1016/0008-8749(88)90310-3. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Roy S., Wainberg M. A. Role of the mononuclear phagocyte system in the development of acquired immunodeficiency syndrome (AIDS). J Leukoc Biol. 1988 Jan;43(1):91–97. doi: 10.1002/jlb.43.1.91. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Stoddart C. A., Scott F. W. Isolation and identification of feline peritoneal macrophages for in vitro studies of coronavirus-macrophage interactions. J Leukoc Biol. 1988 Nov;44(5):319–328. doi: 10.1002/jlb.44.5.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E., Groh V., Popovic M., Mann D. L., Konrad K., Safai B., Eron L., diMarzo Veronese F., Wolff K., Stingl G. Epidermal Langerhans cells--a target for HTLV-III/LAV infection. J Invest Dermatol. 1987 Feb;88(2):233–237. doi: 10.1111/1523-1747.ep12525402. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]





