Abstract
Schizophrenia may be associated with a fundamental disturbance in the temporal coordination of information processing in the brain, leading to classic symptoms of schizophrenia such as thought disorder and disorganized and contextually inappropriate behavior. Despite the growing interest and centrality of time-dependent conceptualizations of the pathophysiology of schizophrenia, there remains a paucity of research directly examining overt timing performance in the disorder. Accordingly, the present study investigated timing in schizophrenia using a well-established task of time perception. Twenty-three individuals with schizophrenia and 22 non-psychiatric control participants completed a temporal bisection task, which required participants to make temporal judgments about auditory and visually presented durations ranging from 300 to 600 ms. Both schizophrenia and control groups displayed greater visual compared to auditory timing variability, with no difference between groups in the visual modality. However, individuals with schizophrenia exhibited less temporal precision than controls in the perception of auditory durations. These findings correlated with parameter estimates obtained from a quantitative model of time estimation, and provide evidence of a fundamental deficit in temporal auditory precision in schizophrenia.
Keywords: schizophrenia, timing, temporal processing, temporal coordination, variability
Schizophrenia may be associated with a fundamental disturbance in the temporal coordination of information processing in the brain, leading to dysfunctions in the timing of perceptual, cognitive, and motor processes, and disturbances of consciousness (Andreasen et al., 1998; Bressler, 2003; Paulus & Braff, 2003; Phillips & Silverstein, 2003; Tononi & Edelman, 2000). This putative deficit in the temporal coordination of information processing in the brain is sometimes referred to as cognitive dysmetria (Andreasen et al.,1998). “Disconnection” models (e.g., Friston, 1998; McGlashan & Hoffman, 2000) posit that deficits in neural communications arise from aberrations in the modulation of time-dependent changes in synaptic connectivity, which manifest as dysfunctions in the timing or sequencing of mental activity and behavior. Accordingly, classic symptoms of schizophrenia such as thought disorder and disorganized and contextually inappropriate behavior may be manifestations of a timing dysfunction (c.f., Andreasen et al., 1998). Support for these conceptualizations is emerging with evidence that brain structures and neurotransmitter systems that are directly linked to neural timing processes are also impaired in schizophrenia (Andreasen, 1999; Andreasen et al., 1998; Rao et al., 1997, 2001; Volz et al., 2001). Despite the growing interest and centrality of these time-dependent conceptualizations of the pathophysiology of schizophrenia, there remains a paucity of research directly examining overt timing performance in the disorder. Thus the aim of the present study was to investigate timing in schizophrenia using a well-established task of time perception.
The temporal relationships between events in the natural environment can be characterized by fixed intervals and/or dynamic flexibility, making the proper encoding of time—including stimulus duration, rate changes, and rhythms—a fundamental prerequisite for everyday perception, attention, and cognition (Barnes & Reiss Jones, 2000; Large & Reiss Jones, 1999; Reiss Jones et al., 2002; Zakay & Block, 1996).
Following this line of thinking, and given that schizophrenia is characterized by profound disturbances in perception, attention, and cognition, it is not surprising that schizophrenia has also been associated with systematic distortions of time (Densen, 1977; Johnson & Petzel, 1971; Tysk, 1983a, 1983b, 1990; Volz et al., 2001; Wahl & Sieg, 1980). Specifically, individuals diagnosed with schizophrenia have consistently been found to experience time as lengthened relative to objective time, which has been interpreted to reflect an increase in the speed of a hypothetical “internal clock” (Densen, 1977; Johnson & Petzel, 1971; Lhamon & Goldstone, 1956; Tracy et al., 1998; Tysk, 1983a, 1983b, 1990; Wahl & Sieg, 1990). For instance, numerous behavioral studies have reported overestimations and underproductions of temporal durations ranging from several seconds to one minute in schizophrenia (Densen, 1977; Johnson & Petzel, 1971; Tysk, 1983a, 1983b, 1990; Volz et al., 2001; Wahl & Sieg, 1980), which is consistent with patients’ subjective reports of an elongated experience of time (Freedman, 1974).
Of further interest is the link between brain regions directly implicated in internal timing processes and those implicated in the pathophysiology of the disorder (Andreasen, 1999; Andreasen et al., 1998; Rao et al., 1997, 2001; Volz et al., 2001). For example, schizophrenia has been associated with deficits of neural communication within cortical-cerebellar (Andreasen, 1999; Andreasen et al., 1998) and cortical-striatal (Volz et al., 2001) brain circuits, which are involved in the temporal coordination of motor activity (Rao et al., 1997, 2001) and the encoding and explicit representation of temporal information (Rao et al., 2001; Voltz et al., 2001), respectively. In addition, dopamine – a neurotransmitter linked to the manifestation of schizophrenia (Carlsson et al., 2001) – has been shown to modulate temporal processing within the cortical-striatal network (Hinton & Meck, 1997; Matell & Meck, 2000). Taken together, it is important to determine the functional integrity of temporal processing in schizophrenia given (i) the essential link between temporal encoding and healthy functioning, (ii) behavioral evidence indicative of a hyperactive internal clock in schizophrenia, and (iii) the overlap between neural circuits implicated in timing processes and those which have been associated with biological and clinical manifestations of the disorder.
Despite behavioral and neuropathological evidence for timing deficits in schizophrenia, the majority of studies of time estimation in schizophrenia have employed durations in the range of several seconds, requiring higher cognitive processes beyond initial sensory registration for temporal encoding (Fraisse, 1984; Michon, 1985; Rammsayer & Lima, 1991). For instance, studies of human timing have indicated that accurate processing of temporal intervals in the range of several seconds requires increased attentional and mnemonic demands (Fortin, 1999; Fortin et al., 1993; Zakay & Block, 1996), making it difficult to delineate deficits of temporal perception from generalized impairments of attention (Kimble et al., 2000; Nestor & O’Donnell, 1998) and memory (Chen & McKenna, 1996) commonly associated with schizophrenia. In the only previously published study of temporal bisection in schizophrenia, Elvevåg and colleagues (2003) addressed this difficulty by employing brief stimuli in the range of milliseconds to better isolate timing mechanisms while minimizing the role of confounding cognitive processes. They found individuals diagnosed with schizophrenia to be less accurate in their timing judgments than non-psychiatric controls, suggesting deficits specific to timing processes. Expanding on the work of Elvevåg and colleagues (2003), the present study is the first to use a within-participant design to examine both auditory and visual bisection in schizophrenia with comparable experimental procedures and durations of the auditory and visual stimuli.
Specifically, the present study aimed to replicate and expand on the findings of Elvevåg and colleagues (2003) in the following ways. First, a temporal bisection task was used to assess the timing of brief durations (i.e., 300–600 ms) in participants diagnosed with schizophrenia and non-psychiatric control participants. The temporal bisection procedure requires participants to first encode short and long anchor durations to which intermediate durations are subsequently compared and classified as closer to either the short or the long anchor. The bisection point therefore refers to the duration at which short and long classifications are made with equal probability. A second feature of the present study was the inclusion of both auditory and visual timing signals within the same experimental session to better examine the effects of attention on duration classification. The observation that sounds are judged longer than visual stimuli of the same objective duration is a robust finding in the timing literature (e.g., Behar & Bevan, 1961; Goldstone et al., 1959; Goldstone & Lhamon, 1972, 1974; Lustig & Meck, 2001; Penney et al., 1998, 2000; Wearden et al., 1998) that has been interpreted to reflect greater attentional allocation to the auditory versus visual signal (Penney et al., 1998, 2000; 2005). Thus, the inclusion of a dual modality bisection task permitted further investigation of the role of attention on temporal processing in schizophrenia, as impaired attention should produce a greater auditory/visual timing difference compared to that observed for non-psychiatric participants.
Finally, a quantitative model of Scalar Timing Theory, which was developed to account for auditory and visual timing differences, was applied to the behavioral data (Penney et al., 1998, 2000). Scalar Timing Theory is a mathematical description of interval timing that attributes variability in temporally-mediated behavioral to different stages of information processing (e.g., Gibbon, 1977, 1991; Gibbon & Church, 1984). In the Sample Known Exactly-Mixed Memories (SKE-MM) model of temporal bisection (Penney et al., 1998, 2000), variability can be introduced during the clock, memory, or decisional stage of temporal processing (see Figure 1; Gibbon et al., 1984). The conversion of real time into subjective time occurs during the clock stage, where a Poisson-variable pacemaker is assumed to emit pulses that get stored in the accumulator during a timed interval. A stimulus signaling the start of the interval causes an attention-modulated switch to close, which allows the pulses to flow into the accumulator where they are summed upon termination of the timed duration. The summation of pulses forms a subjective time estimate that is passed to working memory and compared with previously timed durations that are sampled from reference memory during the decisional stage. The SKE-MM model therefore includes parameters associated with both perceptual (i.e., clock stage) and higher order (i.e., memory and decisional stages) cognitive processes, thus permitting a quantitative attribution of group differences to attentional and/or memory and decisional factors.
Figure 1.
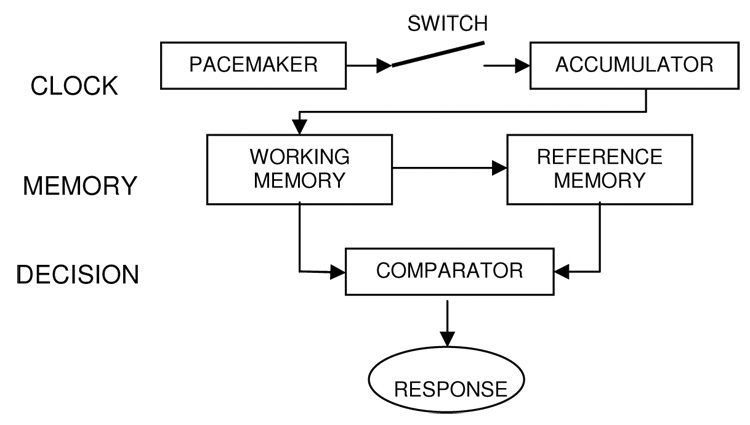
Schematic representation of the three-stage internal clock model.
The hypotheses for the present study were as follows: Consistent with the findings of Elvevåg and colleagues (2003), individuals diagnosed with schizophrenia were predicted to show greater overall timing variability compared to non-psychiatric participants. In a previous study of mixed-modality temporal bisection, the offspring of individuals with schizophrenia exhibited an increased difference in their temporal judgments between auditory and visual signals compared with both controls and individuals identified as high-risk for the development of affective disorders (Penney et al., 2005). Based on these findings and reports of auditory/visual differences observed using a variety of patient populations (Ehrensing et al., 1970; Goldstone & Kirkham, 1968; Goldstone & Lhamon, 1972; Lustig & Meck, 2001) and temporal discrimination tasks (Goldstone, 1968; Goldstone & Goldfarb, 1964; Goldstone et al., 1959), it was predicted that individuals with schizophrenia would judge the auditory stimuli as longer than visual stimuli of the same objective duration, and that this auditory/visual difference would be greater than that observed for the non-psychiatric participants. Because this is the first study to date to apply the SKE-MM model to bisection data from individuals diagnosed with schizophrenia, the model was applied as part of an exploratory analysis.
Methods
Participants
Twenty-three (15 male: mean age = 37.6, SD = 11.6; 8 female: mean age = 43.6, SD = 9.3) individuals meeting DSM-IV (American Psychiatric Association, 1994) criteria for schizophrenia and 22 (6 male: mean age = 34.0, SD = 9.1; 16 female: mean age = 43.8, SD = 12.2) non-psychiatric healthy participants volunteered for the present study. Each of the schizophrenia participants was evaluated using the Structured Clinical Interview for the DSM-IV (Diagnostic and Statistical Manual IV) for Axis I disorders (SCID-I; First et al., 1995), supplemented by chart information. All control participants were interviewed using the SCID-NP (Spitzer et al., 1990) to exclude individuals with Axis I disorders, antisocial personality disorder, and schizophrenia spectrum personality disorders. The patient sample was recruited through outpatient and inpatient units at community and state hospitals and comparison participants were recruited via newspaper advertisement. Exclusion criteria for all participants included self-reported neurological disease, head injury resulting in loss of consciousness for more than 5 minutes, and personal or family history of schizophrenia in the non-psychiatric group. All participants had normal or corrected-to-normal vision and hearing acuity. Groups did not significantly differ with regard to age (schizophrenia: mean age = 39.7, SD = 11.0; control: mean age = 41.1, SD = 12.1), and although educational attainment was significantly greater in the non-psychiatric participants (mean years = 14.3, SD = 2.5) compared to the patient sample (mean years = 11.1, SD = 1.7), t(31) = 4.51, p < .001, Pearson correlations computed within each group revealed no significant relationships between years of education and any of the bisection measures. Indiana University’s Human Subjects Institutional Review Board approved of this study and written informed consent was obtained from all participants.
Current symptom levels in the patient group were assessed by trained diagnosticians using the Positive and Negative Syndrome Scale (PANSS; Kay, et al., 1987). Symptom ratings were categorized into a five-factor structure (i.e., positive, negative, cognitive, hostility, and emotional discomfort) that has been suggested to provide a more sensitive subtype classification than the conventional Positive, Negative, and General subscale distribution (Bell et al., 1994a, 1994b; Kay & Sevy, 1990).
At the time of testing, 15 patients with schizophrenia were taking antipsychotic medications (2 = typical antipsychotics, 9 = atypical antipsychotics, 4 = both typical and atypical antipsychotics), 1 patient was not medicated, and the medication status of 7 patients could not be determined due to unavailable information or randomization in a double-blind pharmaceutical study. Of the 15 medicated participants, 5 were also receiving anticholinergic medication, 3 were receiving antidepressant medication, and 5 were taking adjunctive medications (e.g., ativan, depakote, neurontin). Chlorpromazine equivalents were computed for patients receiving antipsychotic medication, and the resulting dosages were correlated with all behavioral measures to assess for medication effects on timing performance. No significant relationships were noted.
Temporal Stimuli
For both auditory and visual modalities, anchor durations consisted of 300 ms and 600 ms stimuli, with five arithmetically spaced intermediate durations of 350, 400, 450, 500, and 550 ms. Auditory durations were 880 Hz tones produced by GoldWave audio software and presented through Sony headphones. Visual signals consisted of a 3.2 × 3.3 in bird silhouette presented on a computer monitor that was between 40 and 50 cm from the participant’s forehead for the duration of the aforementioned seven stimulus intervals.
Task Procedure
The presentation of the auditory and visual stimuli was randomly determined across trials. Upon presentation of a stimulus, the participant classified it according to its perceived similarity to the short (300 ms) and long (600 ms) anchor values. To address potential difficulties related to task comprehension, a concrete procedural context related to bird classification was adapted from Elvevåg and colleagues (2003).
The task procedure was divided into training, practice, and test phases. The experiment began with the training phase in which the short and long auditory anchor durations were associated with a small (1.84 × 1.92 in) and large (3.60 × 3.79 in) silhouette of a perched bird, respectively, and the short and long visual anchor durations were associated with the silhouette of a flying bird that was presented for the duration of the interval. Participants were told that the auditory tones depict the length of the sound made by the small (300 ms) and large (600 ms) birds, and that the visual durations represent the length of time that the bird flies. To ensure that participants had learned the anchor durations, three presentations of each auditory/visual anchor were randomly administered within a 12-trial practice block. Following presentation of the anchor, participants received on-screen instructions to press the “Short” key if the duration corresponded to the small /short-flying bird and to press the “Long” key if the duration corresponded to the big/long-flying bird. Visual feedback (i.e., “correct” or “incorrect”) was provided after each response, and correct responses were rewarded with a monetary bonus of 10 cents. A 1 s inter-trial interval separated feedback offset and stimulus presentation. The training phase was repeated in 12-trial blocks until an accuracy level of 75% or greater was reached: the session was aborted if 75% accuracy had not been achieved after three blocks.
The test phase of the experiment was presented in three blocks of 70 trials each, with 5 presentations of each auditory/visual duration per block. Participants classified each stimulus as either “Short” or “Long” based on its perceived similarity to the sounds made by the small and large bird (auditory modality) or the flight time corresponding to the short- and long-flying bird (visual modality). Each correct classification of the short and long anchors during the test phase earned participants a reward of 10 cents, for a possible bonus of $6.00. To help ensure that the participants understood the task, a practice phase consisting of one presentation of each stimulus was administered immediately prior to the test phase to allow participants to ask questions and become familiar with the procedure.
Each test block was preceded by a short (< 5 min) rest period. To minimize memory demands for the anchor values, the auditory and visual anchors were presented prior to the commencement of each of the three test blocks.
Behavioral Data and Analysis
The proportion of long responses, p(long), made to the anchors and intermediate signals were quantified separately for each participant and modality. The proportional data can be plotted as a function of signal duration to yield a psychometric response curve that is typically sigmoidal in form, indicating a near absence of long responses to signals that fall close to the short anchor value, to a predominance of long responses as signals come to approach the duration of the long anchor. Sigmoidal functions were fit to the proportional response data from each participant using the regression feature of SigmaPlot (2001, Version 7.0), which employs a least squares method to estimate equation parameters and identify the durations that correspond to p(long) values of 0.25, 0.50, and 0.75 from the fitted sigmoidal curve.
The duration at which the proportion of long responses was equivalent to 0.50 for each modality condition was identified as the bisection point, or the duration at which short and long responses occurred with equal probability. In addition to the bisection point, the values derived from the fitted sigmoidal functions were used to calculate the difference limen (DL) and Weber fraction (WF), which represent the slope of the psychometric response curves and can be interpreted as an index of timing variability. The DL is calculated as one-half the difference between the durations corresponding to p(long) = 0.75 and p(long) = 0.25 ( (0.75 – 0.25) / 2 ), where smaller values indicate steeper slopes and greater temporal precision. The WF is computed by dividing the DL by the bisection point, which normalizes the DL values with respect to the timed durations. Thus, the WF provides an index of Weber’s Law (i.e., a constant coefficient of variation of subjective time across various temporal durations) by allowing for a direct comparison of timing variability across various anchor pairs.
Unless noted, repeated-measures ANOVAs were applied to all analyses. Bisection point, DL, and WF analyses each included Modality (auditory/visual) as a within-subjects factor and Group (schizophrenia/non-psychiatric) as a between-subjects factor. Within each modality, response accuracy to the anchor durations was assessed using Anchor (short/long) as a within-subjects variable and Group (schizophrenia/non-psychiatric) as a between-subjects variable.
Theoretical Model
The Sample-Known Exactly-Mixed Memories (SKE-MM) model of duration bisection (Penney et al., 1998, 2000) consists of four parameters. The degree of noise in the memory distributions is represented by the sensitivity parameter, gamma (γ). The sensitivity parameter is the coefficient of variation of remembered time, and affects the slope of the psychometric bisection function with smaller values (higher sensitivity) indicating steeper slopes. Although not identical to the empirically based Weber ratio, γ can be viewed as a comparable measure of proportionality in time estimations (Gibbon, 1981; Penney et al., 1998). In addition to the sensitivity parameter, the SKE-MM model contains a bias parameter, β, which serves as the threshold value between “short” (RS) and “long” (RL) response alternatives and represents a bias towards RL (i.e., β > 1). The relative rate parameter, RR, is the ratio of the visual clock speed to the auditory clock speed, with values of 1.0 signifying equivalent clock rates and values below 1.0 indicating a faster speed for the auditory clock. Finally, Pa (or 1-Pv), represents the proportional contribution of the auditory signal to the memory mixture. The value of Pa can range from zero to one, specifying zero to complete auditory dominance of the memory distribution, respectively. The mathematical and theoretical foundations, as well as its application to the bisection procedure, are described in detail elsewhere (Allan & Gibbon, 1991; Gibbon, 1981, 1986; Gibbon et al., 1984; Meck, 1996, 2003; Penney et al., 1998, 2000).
The SKE-MM model was fit to individual mean response data from the auditory and visual modalities using Matlab’s (version 6.5, 2002) fminsearch function, which uses a simplex search algorithm to minimize the parameter space. The γ, β, RR, and Pa parameters were allowed to vary, and parameter values from the best fit were recorded and submitted for analysis. Because the SKE-MM model was designed to fit auditory and visual response data simultaneously, the model yields a single value for each of the γ, β, Pa, and RR parameters; hence, parameter values were submitted to separate one-way ANOVAs with a between-subjects factor of Group (schizophrenia/non-psychiatric).
Theoretical Considerations
Pearson product-moment correlations were performed between behavioral and parameter estimates of temporal precision to assess the correspondence between theoretical “clock” accounts of temporal processing and actual timing behavior. Specifically, γ values from the SKE-MM model were correlated with DL and WF measures from the auditory/visual bisection task, with a Bonferroni correction applied for multiple comparisons.
Clinical Considerations
Pearson product-moment correlation coefficients were computed between PANSS symptom ratings and temporal precision (i.e., DL, WF) using a Bonferroni correction for multiple comparisons to assess the relationship between symptom severity and performance variability. To examine the extent to which any differences in temporal performance between the schizophrenia and control groups could be attributed to timing-specific factors rather than to more generalized deficits associated with schizophrenia, available measures of intellectual functioning were correlated with behavioral timing variables. Specifically, the Picture Completion, Digit-Symbol, Similarities, and Digit Span subscale scaled scores from the Wechsler Adult Intelligence Scale (WAIS) and IQ estimates from the 2-subtest Wechsler Abbreviated Scale of Intelligence (WASI) were correlated with bisection point, difference limen, and Weber fraction values within each participant group using a Bonferroni correction for multiple comparisons.
Outlier Considerations
A within-group boxplot method of outlier identification was performed separately for each analysis to classify extreme cases, defined as data values more than six quartiles from the upper or lower ends of the interquartile range. A single extreme case was identified for the DL and WF variables, resulting in the removal of one non-psychiatric participant from both analyses. In addition, one non-psychiatric participant and one patient with schizophrenia were identified as extreme outliers for the Pa and RR parameter, respectively, of the SKE-MM model, resulting in the removal of these participants from all model analyses.
Results of the major dependent variables are reported with their corresponding partial eta2 [ηp2] effect sizes, where small effect sizes are less than .06, moderate effect sizes range from .06 to .14, and large effect sizes are greater than .14 (Cohen, 1973).
Results
Practice Accuracy
Six patients with schizophrenia and two non-psychiatric participants did not complete the auditory/visual task due to an inability to correctly differentiate between the short and long anchors at an accuracy level of 75% after three practice blocks. The remaining 17 patients (M = 83.3, SD = 8.1) and 20 non-psychiatric participants (M = 86.1, SD = 9.5) did not significantly differ with regard to practice accuracy and moved on to the subsequent phases of the task.
Bisection Point
Averaged psychophysical functions for the auditory and visual conditions are presented in Figure 2, which illustrates the proportion of long responses made to the anchors and intermediate durations. The location of the bisection point did not significantly differ between the schizophrenia and non-psychiatric control groups or between the auditory and visual modalities (see Table 1). However, a significant Group × Modality interaction, F(1,42) = 9.84, ηp2 = 0.19, p = .003, captured the differential shift in the location of the bisection point from the auditory to visual condition between patient and comparison participants. Whereas the visual bisection point was shifted to the left of the auditory bisection point in the patient group, the non-psychiatric control participants showed the customary rightward shift of the visual compared to the auditory response curve (Penney et al., 1998, 2000).
Figure 2.
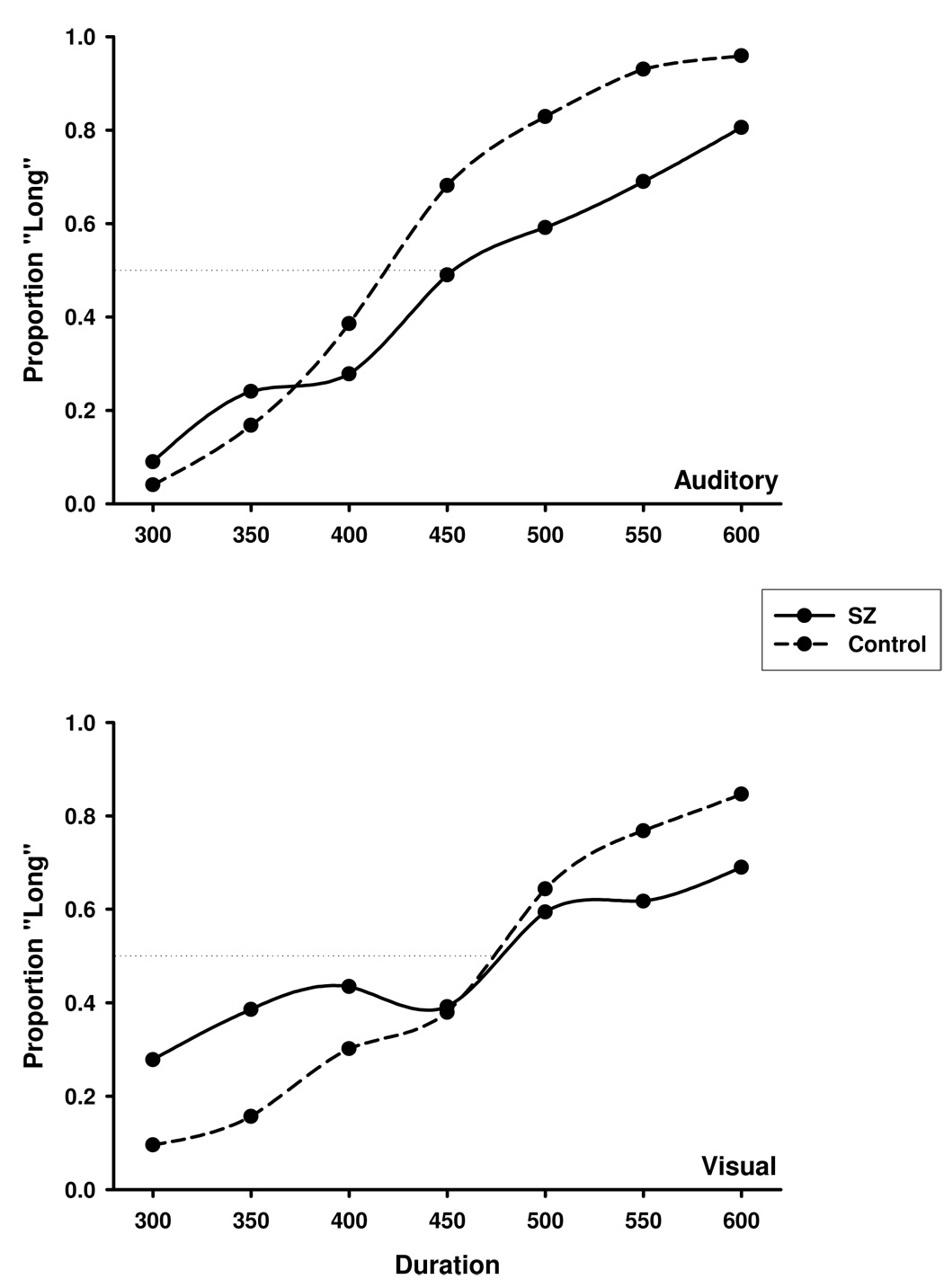
The proportion of long responses as a function of signal duration for the auditory (top) and visual (bottom) modalities. SZ = schizophrenia, Control = non-psychiatric participants.
Table 1.
Bisection Point, Difference Limen, and Weber Fraction Values from Auditory and Visual Modalities for Non-Psychiatric Control Participants and Patients with Schizophrenia (SZ)
| Control | SZ | |||
|---|---|---|---|---|
| Condition | M | SD | M | SD |
| Bisection Point | ||||
| Auditory | 422.12 | 38.77 | 436.17 | 68.37 |
| Visual | 455.30 | 48.39 | 408.52 | 74.29 |
| Difference Limen | ||||
| Auditory | 40.03 | 16.89 | 66.67 | 36.39 |
| Visual | 50.82 | 18.64 | 48.70 | 43.72 |
| Weber Fraction | ||||
| Auditory | 0.091 | 0.034 | 0.138 | 0.076 |
| Visual | 0.100 | 0.043 | 0.088 | 0.091 |
Response Variability
As can be noted in Figure 2, patients appeared to have flatter gradients and less response precision around the anchor durations for both modality conditions compared to the non-psychiatric group (see also Table 1). Analysis of the difference limen supported this observation, indicating a significant main effect of group, F(1,42) = 4.09, ηp2 = 0.09, p = .050. Although no significant differences in DL values were found between the auditory and visual modalities, a trend toward a Group × Modality interaction was noted, F(1,42) = 3.82, p = .057, and associated with a moderate effect size (ηp2 = 0.08). To further explore this trend, post-hoc independent-samples t-tests were performed separately for the auditory and visual conditions. Whereas no group differences were found for visual stimuli, the patient group showed significantly greater response variability than non-psychiatric participants in the auditory modality, t(42) = 2.23, p = .031 (see Table 1).
In contrast to the difference limen, analysis of the Weber fraction indicated no significant differences in response variability between the patient and non-psychiatric groups. The Weber fraction normalizes a given participant’s DL by his or her bisection point, resulting in an intrasubject measure of variance. It has been suggested that intrasubject measures are less sensitive to between-subject effects (Altman, 1991), which may account for the differential results obtained from the DL and WF analyses. Analysis of the Weber fraction also showed no significant effects of modality, though a slight trend towards a Group × Modality interaction was indicated, F(1,42) = 3.38, p = .073, and associated with a moderate effect size (ηp2 = 0.08). Similar to the difference limen, post-hoc independent-samples t-tests revealed a significant group difference for the auditory, t(41) = 2.55, p = .014, but not visual, modality (see Table 1).
Anchor Accuracy
To assess retention of the short and long anchors, classification judgments for the auditory and visual anchors during the test phase of the task were evaluated as a proportion of correct responses. A significant main effect of group with a large effect size indicated that response accuracy was lower for the patients (M = 0.78, SD = 0.02) across all anchor-modality pairs compared to that of the non-psychiatric group (M = 0.94, SD = 0.02), F(1,43) = 30.27, ηp2 = 0.41, p < .001. Modality of presentation yielded a statistically significant effect with a large effect size, indicating decreased accuracy in response to the visual (M = 0.80, SD = 0.02) compared to auditory (M = 0.92, SD = 0.01) anchors, F(1,43) = 34.47, ηp2 = 0.45, p < .001. Finally, a significant anchor effect showed that classification judgments were more accurate in response to the 300 ms (M = 0.89, SD = 0.01) compared to the 600 ms (M = 0.83, SD = 0.02) anchor, F(1,43) = 4.63, ηp2 = 0.10, p = .037.
In addition to overall accuracy, retention of the anchor values was evaluated to assess whether patients showed poorer memory for the anchors across the duration of the experiment. The difference in response accuracy between Block 1 and Block 3 did not significantly differ between patients (M = 0.04, SD = 0.02) and non-psychiatric participants (M = 0.02, SD = 0.02) across all anchor stimuli or within modality. Pearson correlations indicated no significant relationships between anchor accuracy and DL and WF measures of variance in the schizophrenia group or in the controls, suggesting that increased performance variability was not related to poorer retention of the anchor durations.
Theoretical Model
Figure 3 illustrates the obtained and model-predicted response curves from the SKE-MM model for each group-by-modality condition. The parameter values obtained from the auditory and visual conditions are displayed in Table 2. A significant group difference, associated with a large effect size, was found for the sensitivity parameter (γ, gamma), F(1,41) = 29.42, ηp2 = 0.42, p < .001, suggesting that schizophrenia is associated with increased memory variability of the anchor durations. No significant group effects were found for the bias parameter (β), though values below 1.0 in each group indicated a minimal response bias towards signal classifications of short.
Figure 3.
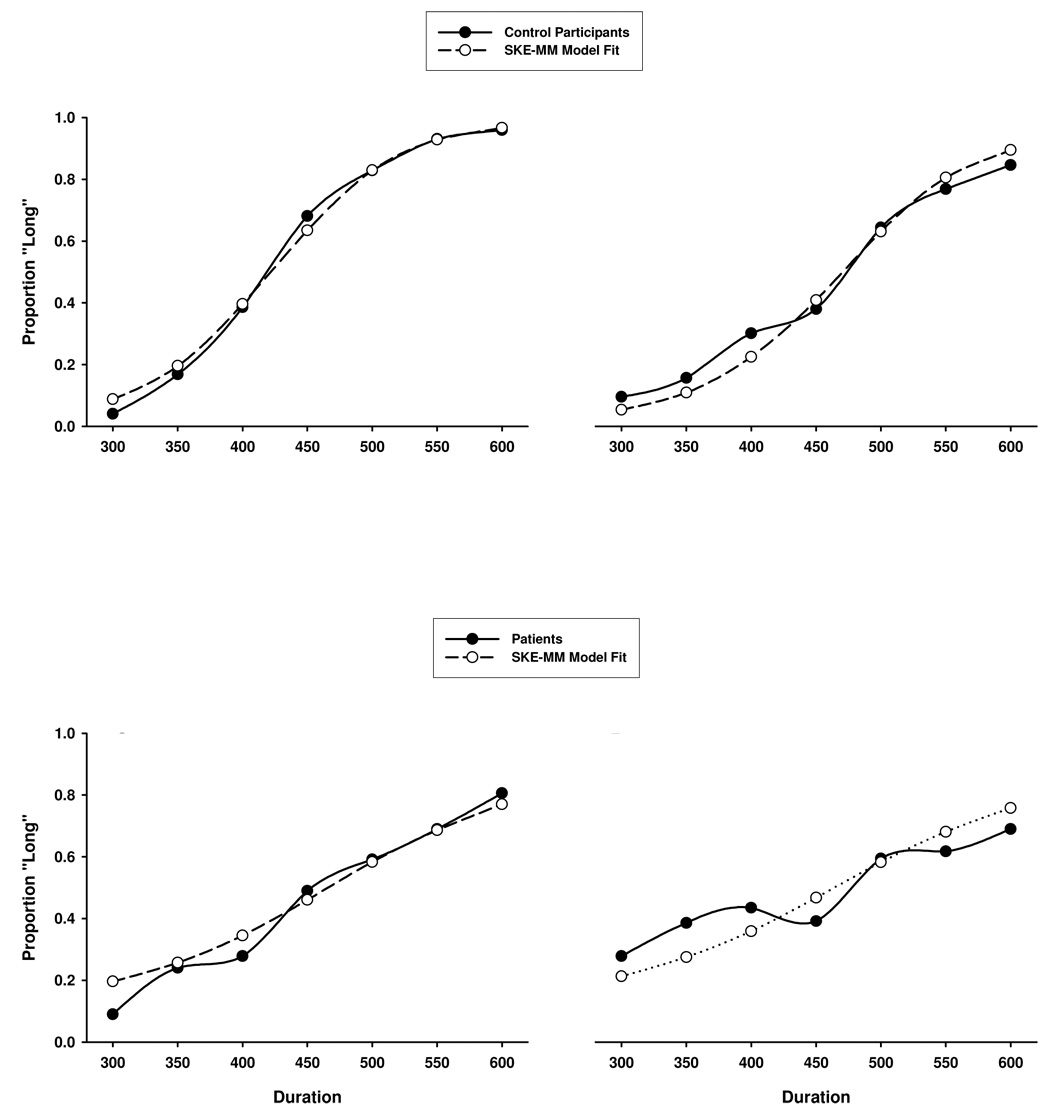
Actual and SKE-MM model-predicted responses for auditory (A and C) and visual (B and D) modalities.
Table 2.
Parameter Values from the SKE-MM Model Obtained for Non-Psychiatric Control Participants and Patients with Schizophrenia (SZ)
| Parameter | Control | SZ | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Gamma (γ) | 0.23 | 0.09 | 0.49 | 0.24 |
| Beta (β) | 0.93 | 0.26 | 0.81 | 0.95 |
| Proportion Auditory (Pa) | 0.82 | 0.13 | 0.43 | 0.4 |
| Relative Rate (RR) | 0.89 | 0.11 | 1.06 | 0.25 |
The Pa parameter, which captures the memory-mixture component of the SKE-MM model, was found to significantly differ between patient and non-psychiatric groups, F(1,41) = 21.16, ηp2 = 0.34, p < .001. As noted in Table 2, non-psychiatric participants evidenced a proportionally greater contribution of the auditory signal to the 300 ms and 600 ms memory mixtures. In contrast, memory distributions were almost equivalently influenced by the auditory and visual signals in the patient group, with a slightly greater influence of the visual modality. A significant group effect was also found for the relative rate parameter, F(1,41) = 7.32, ηp2 = 0.15, p = .010, with Relative Rate values greater than 1.0 in the patient group indicating a faster visual, compared to auditory, clock rate. A mean Relative Rate value of 0.89 for the comparison sample is more closely related to previous reports of an auditory clock that runs approximately 10% faster than its visual counterpart (Penney et al., 1998, 2000).
Theoretical Considerations
To assess the relationship between behavioral and parameter estimates of temporal precision, difference limen and Weber fraction indices of response variability from the auditory and visual modalities were correlated with gamma estimates of timing variability from the SKE-MM model. Auditory difference limen (DL) and Weber fraction (WF) values significantly correlated with estimated gamma values from the SKE-MM model for both patient (DL: r(23) = 0.58, p = .004; WF: r(23) = 0.62, p = .002) and non-psychiatric (DL: r(20) = 0.60, p = .006; WF: r(20) = 0.55, p = .013) groups. In contrast, no significant relationships were revealed between gamma parameter estimates and visual difference limen and Weber fraction values for either patients or non-psychiatric participants.
Clinical Considerations
Positive (M = 15.5, SD = 6.3), negative (M = 16.2, SD = 5.7), cognitive (M = 15.1, SD = 5.4), hostility (M = 6.3, SD = 3.3), and emotional discomfort (M = 7.7, SD = 3.2) symptom ratings from the PANSS were not significantly related to measures of timing from the temporal bisection (i.e., DL, WF) task (see Table 3). Although the Picture Completion (SZ: M = 5.96, SD = 1.94; control: M = 10.72, SD = 3.10; t(39) = 6.05, p < .001), Digit-Symbol (SZ: M = 6.26, SD = 1.79; control: M = 11.83, SD = 2.60; t(39) = 8.13, p < .001), Similarities (SZ: M = 6.35, SD = 4.63; control: M = 10.56, SD = 2.64; t(39) = 3.44, p = .001), and Digit Span (SZ: M = 7.35, SD = 2.84; control: M = 12.11, SD = 2.72; t(39) = 5.43, p < .001) subscales from the WAIS and IQ estimates from the WASI (SZ: M = 82.94, SD = 14.10; control: M = 108.61; SD = 10.92; t(33) = 6.04, p < .001) significantly differed between the schizophrenia and non-psychiatric groups, a statistically significant relationship was only observed between Picture Completion subscale scores and visual bisection point values in the schizophrenia group (r(23) = 0.59, p = .003)
Table 3.
Pearson Product-Moment Correlation Coefficients Between Symptom Ratings and Behavioral Indices of Temporal Precision
| Positive And Negative Symptom Scale Factors | ||||||
|---|---|---|---|---|---|---|
| Positive | Negative | Cognitive | Hostility | Emotion | ||
| Temporal Bisection | ||||||
| Difference | Auditory | −0.215 | 0.098 | −0.167 | −0.383 | 0.011 |
| Limen | Visual | 0.32 | 0.293 | 0.274 | 0.223 | 0.325 |
| Weber | Auditory | −0.051 | 0.317 | −0.108 | −0.264 | 0.06 |
| Fraction | Visual | 0.381 | 0.212 | 0.087 | 0.099 | 0.147 |
Discussion
The combined use of behavioral timing methods and quantitative modeling to investigate time perception in schizophrenia provided a means of assessing specific aspects of temporal processing associated with the timing of extremely short durations in both auditory and visual modalities. Whereas behavioral variables such as the bisection point and measures of variability (i.e., DL, WF) provided descriptive measures of temporal performance, the application of a theoretical model based on the laws of Scalar Timing Theory allowed for a more rigorous assessment of behavior by dissociating temporal processing into clock, memory and decisional stages (see Figure 1). Consistent with previous behavioral findings (Brown et al., 2005; Goldstone, 1975; Lhamon & Goldstone, 1973; Tysk, 1983a, 1983b), individuals with schizophrenia exhibited less temporal precision (i.e., flatter response gradients) than non-psychiatric controls in the perception of auditory durations. This increased performance variability in schizophrenia was unlikely due to more generalized dysfunctions associated with illness severity as suggested by the absence of significant relationships between symptom and intelligence scales and behavioral indices of temporal precision. Mnemonic failure associated with retention of the anchor durations across the task is also unlikely to account for the greater auditory timing variability observed in schizophrenia, as memory for the auditory anchors did not differ between the schizophrenia and control groups. Both schizophrenia and non-psychiatric groups displayed greater temporal variance in the visual compared to the auditory modality. Similar to auditory timing, statistically equivalent retention rates of the visual anchors between groups suggested that visual timing performance in schizophrenia was not unduly influenced by memory requirements inherent in the temporal bisection task. Taken together, the results provide evidence of a fundamental deficit in temporal auditory precision in schizophrenia, which is manifest as greater temporal response variability.
Bisection point values did not differ between groups across modalities, indicating that individuals with schizophrenia did not systematically over or underestimate durations relative to non-psychiatric control participants. However, individuals with schizophrenia did not show the customary rightward shift of the visual response function. Studies of mixed-modality bisection have consistently shown higher bisection point values for visually presented durations, suggesting that more clock “pulses” are needed for visual than auditory stimuli to perceive the same objective duration (Penney et al., 1998, 2000). Whereas non-psychiatric participants displayed a rightward shift of the visual bisection point, a leftward shift of the visual response function (i.e., a lower bisection point) was observed in the schizophrenia group.
The leftward shifted response function suggests that patients judged the visual stimuli as longer than the auditory tones of the same duration. This observed behavioral response pattern was corroborated by parameter estimates from the SKE-MM model, with a relative rate value of 1.06 indicating a 6% increase in the speed of the visual relative to the auditory clock. In contrast, relative rate parameter estimates in the non-psychiatric group were consistent with previous reports of a 10% increase in auditory relative to visual clock speed (Penney et al., 1998, 2000). The proportional contribution of the auditory modality to the anchor values stored in memory (i.e., Pa parameter) is related to relative rate values in the SKE-MM model, representing the degree of “shift” in the auditory and visual response function. Specifically, Pa values correspond to the proportion of the reference memory mixture dominated by the auditory signal. If a single modality dominates the memory mixture, a shift would only be expected in the non-dominant response function.
For instance, previous human studies have reported an auditory dominant memory mixture (Penney et al., 1998, 2000). Hence, on visual trials, a comparison is made between a perceived duration encoded at visual clock speed and a memory comparison based on auditory clock speed. If the visual clock is assumed to run slower than the auditory clock, the visual function would show the customary rightward shift with no change in the auditory response function. In contrast, memory mixtures equally composed of the auditory and visual signals would be expected to produce a shift in both response functions, with the direction of the shift dependent on the individual clock speeds. Consistent with previous studies, Pa values from the non-psychiatric group indicated a proportionally greater contribution of the auditory signal to the anchor memories. By comparison, memory distributions were almost equivalently influenced by the auditory and visual signals in the patient group, with a slightly greater influence of the visual modality.
Taken together, the relative rate and proportional auditory contribution parameter estimates from the SKE-MM model add explanatory power to the observed differences in the auditory and visual bisection points. Specifically, the leftward shift of the visual bisection point in the schizophrenia group may be attributed to a faster visual clock. Furthermore, the non-dominant memory mixture found for individuals with schizophrenia suggests that duration estimates for both modalities were shifted relative to estimates that would be made in a single-modality task. Finally, the absence of group differences in response bias values rules out the possibility that the differential modality shifts between patients and non-psychiatric participants were due to decisional biases.
A faster visual clock in schizophrenia is contrary to prediction, as the increased attentional allocation needed to encode visual stimuli, along with evidence for early/basic visual processing deficits in schizophrenia (e.g., O’Donnell et al., 2006), would suggest greater difficulty in the timing of visual versus auditory durations (Penney et al., 1998, 2000, 2005). These results are also in contrast to previous reports of mixed-modality bisection in the offspring of schizophrenia patients (Penney et al., 2005). Compared to non-psychiatric participants, the offspring of patients showed a larger rightward shift of the visual response function. Furthermore, application of the SKE-MM model suggested that the enhanced discrepancy between the auditory and visual modalities was due to disturbances of attention on visual clock speed (Penney et al., 2005). The inconsistency in findings between the present study and that of Penney and colleagues (2005) may be attributed to a number of factors including duration length (300–600 ms in the present study vs. 3–6 s in Penney et al.), clinical severity (symptomatic patients in the present study vs. at-risk individuals in Penney et al.), and the potential influence of antipsychotic medication on temporal performance in the present study. Differences in task complexity may also account for the inconsistent findings between studies. For example, the bisection task employed by Penney and colleagues (2005) included single modality trials (as in the present study), but these trials were randomly intermixed with trials during which asynchronously presented auditory and visual signals overlapped in time. In addition to allocating more attentional resources to the timing of simultaneous signals, it is likely that greater attentional resources were also employed during the single modality trials as a result of anticipation of the second signal. Despite these differences, it remains unclear why patients in the present study showed a leftward rather than rightward shift of the visual relative to the auditory response function. Repeated evidence for deficits of attention associated with schizophrenia (e.g., Kimble et al., 2000; Nestor & O’Donnell, 1998) suggests that the leftward shift of the visual response curve found for individuals with schizophrenia is unlikely the result of increased attention during visual timing, and highlights the importance of continued research on temporal processing in the disorder.
Also contrary to prediction was the absence of group differences in temporal variance for the visual modality. Because slower clock speeds have been associated with greater timing variability (Goldstone, 1975; Lhamon & Goldstone, 1973; Stanford & Santi, 1998), one would expect that decreased visual clock speed, and hence, increased visual response variability, would be observed in schizophrenia. When considering the obtained pattern of results, however, the absence of group differences in visual timing variance is consistent with the increased visual clock speed, and hence decreased visual response variability, found for the schizophrenia group. Accordingly, greater auditory timing variability was found for individuals with schizophrenia compared to the non-psychiatric participants, which corresponds to the slower auditory compared to visual clock rates observed in the schizophrenia group.
Consistent with increased behavioral indices of auditory timing variability in the schizophrenia group, gamma estimates of variability from the SKE-MM model indicated decreased temporal precision for individuals with schizophrenia compared to non-psychiatric participants. Importantly, gamma values were found to correlate only with behavioral measures of auditory variance for both groups, with no meaningful relationships observed between gamma and indices of visual timing variability. The specificity of gamma to the auditory modality for both patients and non-psychiatric participants suggests that auditory memory variance may account for much of the observed variability in duration judgments.
To date, this is the first study that has examined mixed-modality bisection and successfully applied the SKE-MM model to individuals with schizophrenia. Application of the SKE-MM model provides a means of quantitatively differentiating temporal performance into clock, mnemonic, and decisional processes. At the level of the clock, the relative rate parameter indicated a 6% increase in the speed of the visual relative to the auditory clock in individuals with schizophrenia, which was in contrast to the 10% increase in the auditory relative to visual clock speed observed in the non-psychiatric group. Gamma estimates of temporal memory variability suggested that memories for the anchor durations may be less precise in individuals with schizophrenia. Also at the mnemonic level, estimates of the proportional contribution of the auditory modality to the anchor memories (i.e., Pa) indicated auditory dominant memories in non-psychiatric participants, but relatively equivalent contributions of the auditory and visual modalities to the anchor memories in schizophrenia. Finally, estimates of response bias (β) indicated a minimal bias towards signal classifications of short at the decisional level in both schizophrenia and non-psychiatric groups. Taken together, findings from the SKE-MM model suggest that disturbances in the timing of extremely short durations in schizophrenia may arise at the clock and memory stages of temporal processing. Examination of the relationships between the behavioral and model indices of bisection performance further elucidated the model data, indicating that the timing of visual, rather than auditory, durations may be primarily responsible for the clock and memory disturbances observed in schizophrenia.
To better understand potential differences between auditory and visual temporal bisection in schizophrenia, it will be important for future studies to examine the timing of auditory and visual durations in combined as well as separate test sessions, and to apply both behavioral and quantitative modeling methods to allow for a more rigorous interpretation of temporal processing than that obtained from descriptive measures alone. It will also be important for future studies to control for potential gender differences in the examination of timing in schizophrenia given evidence for differential impairments in proper brain lateralization between male and female individuals with schizophrenia (DeLisi et al., 1989; Walder et al., 2007) and support for the role of the right temporal lobe in decisional processes associated with temporal discrimination (Melgire et al., 2005). Finally, to better account for the role of symptom severity and/or symptom type in temporal performance, it will be of interest to examine timing processes in individuals at different phases of schizophrenia as well as individuals with different symptom profiles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan LG, Gibbon J. Human bisection at the geometric mean. Learning and Motivation. 1991;22:39–58. [Google Scholar]
- Altman DG. Practical statistics for medical research. 1st ed. Chapman & Hall/CRC; 1991. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia. Bleuler’s “fragmented phrene” as schizencephaly. Archives of General Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Barnes R, Reiss Jones M. Expectancy, attention, and time. Cognitive Psychology. 2000;41:254–311. doi: 10.1006/cogp.2000.0738. [DOI] [PubMed] [Google Scholar]
- Behar I, Bevan W. The perceived duration of auditory and visual intervals: Cross-modal comparison and interaction. American Journal of Psychology. 1961;74:17–26. [PubMed] [Google Scholar]
- Bell MD, Lysaker PH, Beam-Goulet JL, Milstein RM, Lindenmayer JP. Five-component model of schizophrenia: Assessing the factorial invariance of the positive and negative syndrome scale. Psychiatry Research. 1994a;52:295–303. doi: 10.1016/0165-1781(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Bell MD, Lysaker PH, Milstein RM, Beam-Goulet JL. Concurrent validity of the cognitive component of schizophrenia: relationship of PANSS scores to neuropsychological assessments. Psychiatry Research. 1994b;54:51–58. doi: 10.1016/0165-1781(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Bressler SL. Cortical coordination dynamics and the disorganization syndrome in schizophrenia. Neuropsychopharmacology. 2003;28 Suppl. 1:35–39. doi: 10.1038/sj.npp.1300145. [DOI] [PubMed] [Google Scholar]
- Brown SM, Kieffaber PD, Vohs JL, Carroll CA, Tracy JA, Shekhar A, O’Donnell BF, Steinmetz JE, Hetrick WP. Eye-blink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain and Cognition. 2005;58:94–108. doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: New evidence. Annual Review of Pharmacology & Toxicology. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Chen EYH, McKenna PJ. Memory dysfunction in schizophrenia. In: Pantelis C, Nelson HE, Barnes TRE, editors. Schizophrenia: A neuropsychological perspective. John Wiley & Sons Ltd.; 1996. pp. 107–124. [Google Scholar]
- Cohen J. Eta-Squared and and Partial Eta-Squared in Communication Science. Human Communication Research. 1973;28:473–490. [Google Scholar]
- DeLisi LE, Dauphinais ID, Hauser P. Gender differences in the brain: Are they relevant to the pathogenesis of schizophrenia? Comprehensive Psychiatry. 1989;30:197–208. doi: 10.1016/0010-440x(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Densen ME. Time perception in schizophrenia. Perception and Motor Skills. 1977;44:436–438. doi: 10.2466/pms.1977.44.2.436. [DOI] [PubMed] [Google Scholar]
- Ehrensing RH, Stokes PE, Pick GR, Goldstone S, Lhamon WT. Effect of alcohol on auditory and visual time perception. Quarterly Journal of the Study of Alcohol. 1970;31:851–860. [PubMed] [Google Scholar]
- Elvevåg B, McCormack T, Gilbert A, Brown GDA, Weinberger DR, Goldberg TE. Duration judgments in patients with schizophrenia. Psychological Medicine. 2003;33:1249–1261. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders (SCID) Washington, DC: Psychiatry Press; 1994. [Google Scholar]
- Fortin C. Short-term memory in time interval production. International Journal of Psychology. 1999;34:308–316. [Google Scholar]
- Fortin C, Rousseau R, Bourque P, Kirouac E. Time estimation and concurrent nontemporal processing: Specific interference from short-term memory demands. Perceptual Psychophysiology. 1993;53:536–548. doi: 10.3758/bf03205202. [DOI] [PubMed] [Google Scholar]
- Fraisse P. Perception and estimation of time. Annual Review of Psychology. 1984;35:1–36. doi: 10.1146/annurev.ps.35.020184.000245. [DOI] [PubMed] [Google Scholar]
- Freedman BJ. The subjective experience of perceptual and cognitive disturbances in schizophrenia. Archives of General Psychiatry. 1974;30:333–340. doi: 10.1001/archpsyc.1974.01760090047008. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophrenia Research. 1998;30:115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychological Review. 1977;84:279–325. [Google Scholar]
- Gibbon J. On the form and location of the psychometric bisection function for time. Journal of Mathematical Psychology. 1981;24:58–87. [Google Scholar]
- Gibbon J. The structure of subjective time: How time flies. In: Bower G, editor. The psychology of learning and motivation. New York: Academic Press; 1986. pp. 105–135. [Google Scholar]
- Gibbon J. Origins of scalar timing. Learning and Motivation. 1991;22:3–38. [Google Scholar]
- Gibbon J, Church RM. Sources of variance in an information processing theory of timing. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal cognition. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 465–488. [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. In: Gibbon J, Allen L, editors. Timing and time perception. New York: Academy of Sciences; 1984. pp. 58–87. [DOI] [PubMed] [Google Scholar]
- Goldstone S. Production and reproduction of duration: Intersensory comparisons. Perceptual and Motor Skills. 1968;26:755–760. doi: 10.2466/pms.1968.26.3.755. [DOI] [PubMed] [Google Scholar]
- Goldstone S. The variability of temporal judgment in psychopathology. In: Keitzman ML, Sutton S, Zubin J, editors. Experimental approaches to psychopathology. NY: Academic Press; 1975. pp. 393–419. [Google Scholar]
- Goldstone S, Boardman WK, Lhamon WT. Intersensory comparisons of temporal judgments. Journal of Experimental Psychology. 1959;4:243–248. doi: 10.1037/h0040745. [DOI] [PubMed] [Google Scholar]
- Goldstone S, Goldfarb JL. Direct comparison of auditory and visual durations. Journal of Experimental Psychology. 1964;67:483–485. doi: 10.1037/h0046997. [DOI] [PubMed] [Google Scholar]
- Goldstone S, Kirkham JE. The effects of secobarbital and dextroamphetamine upon time judgment: Intersensory factors. Psychopharmacologia. 1968;13:65–73. doi: 10.1007/BF00401619. [DOI] [PubMed] [Google Scholar]
- Goldstone S, Lhamon WT. Auditory-visual differences in human temporal judgment. Perceptual and Motor Skills. 1972;34:623–633. doi: 10.2466/pms.1972.34.2.623. [DOI] [PubMed] [Google Scholar]
- Goldstone S, Lhamon WT. Studies of auditory-visual differences in human time judgment: Sounds are judged longer than lights. Perceptual and Motor Skills. 1974;39:63–82. doi: 10.2466/pms.1974.39.1.63. [DOI] [PubMed] [Google Scholar]
- Hinton SC, Meck WH. How time flies: Functional and Neural Mechanisms of Interval Timing. In: Bradshaw CM, Szabadi E, editors. Time and behavior: Psychological and neurobehavioral analyses. New York: Elsevier Science B.V.; 1997. [Google Scholar]
- Johnson JE, Petzel TP. Temporal orientation and time estimation in chronic schizophrenia. Journal of Clinical Psychology. 1971;27:194–196. doi: 10.1002/1097-4679(197104)27:2<194::aid-jclp2270270210>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kay SR, Sevy S. Pyramidical model of schizophrenia. Schizophrenia Bulletin. 1990;16:537–545. doi: 10.1093/schbul/16.3.537. [DOI] [PubMed] [Google Scholar]
- Kimble M, Lyons M, O’Donnell BF, Nestor P, Niznikiewicz M, Toomey R. The effect of family status and schizotypy on electrophysiologic measures of attention and semantic processing. Biological Psychiatry. 2000;47:402–412. doi: 10.1016/s0006-3223(99)00184-5. [DOI] [PubMed] [Google Scholar]
- Large EW, Reiss Jones M. The dynamics of attending: How people track time-varying events. Psychological Review. 1999;106:119–159. [Google Scholar]
- Lhamon WT, Goldstone S. The time sense: Estimation of one second durations by schizophrenic patients. Archives of Neurological Psychiatry. 1956;76:625–629. [PubMed] [Google Scholar]
- Lhamon WT, Goldstone S. Temporal information processing in schizophrenia. Archives of General Psychiatry. 1973;28:44–51. doi: 10.1001/archpsyc.1973.01750310028006. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Paying attention to time as one gets older. Psychological Science. 2001;12(6):478–484. doi: 10.1111/1467-9280.00389. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behavior. Bioessays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Archives of General Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Introduction: Persistence of time. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca Raton, FL: CRC Press; 2003. pp. xvii–xli. [Google Scholar]
- Melgire M, Ragot R, Samson S, Penney TB, Meck WH, Pouthas V. Auditory/visual duration bisection in patients with left or right medial-temporal lobe resection. Brain and Cognition. 2005;58:119–124. doi: 10.1016/j.bandc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Michon JA. The complete time experience. In: Michon JA, Jackson JL, editors. Time, mind, and behavior. Berlin: Springer; 1985. pp. 21–52. [Google Scholar]
- Nestor PG, O’Donnell BF. The mind adrift: Attentional dysregulation in schizophrenia. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: The MIT Press; 1998. pp. 527–546. [Google Scholar]
- O’Donnell BF, Bismark A, Hetrick WP, Bodkins M, Vohs JL, Shekhar A. Early stage vision in schizophrenia and schizotypal personality disorder. Schizophrenia Research. 2006;86:89–98. doi: 10.1016/j.schres.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Braff DL. Chaos and schizophrenia : Does the method fit the madness ? Biological Psychiatry. 2003;53:3–11. doi: 10.1016/s0006-3223(02)01701-8. [DOI] [PubMed] [Google Scholar]
- Penney TB, Allen LG, Meck WH, Gibbon J. Memory mixing in duration bisection. In: Rosenbaum DA, Collyer CE, editors. Timing of behavior: Neural, psychological, and computational perspectives. Cambridge, MA: The MIT Press; 1998. pp. 165–193. [Google Scholar]
- Penney TB, Gibbon J, Meck WH. Differential effects of auditory and visual signals on clock speed and temporal memory. Journal of Experimental Psychology: Human Perception and Performance. 2000;26:1770–1787. doi: 10.1037//0096-1523.26.6.1770. [DOI] [PubMed] [Google Scholar]
- Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval timing deficits in individuals at high risk for schizophrenia. Brain and Cognition. 2005;58:109–118. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behavioral and Brain Sciences. 2003;26:63–135. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- Rammsayer TH, Lima SD. Duration discrimination of filled and empty auditory intervals: Cognitive and perceptual factors. Perception & Psychophysics. 1991;50:565–574. doi: 10.3758/bf03207541. [DOI] [PubMed] [Google Scholar]
- Rao SM, Harrington DL, Haaland KY, Bobholz JA, Cox RW, Binder JR. Distributed neural systems underlying the timing of movements. Journal of Neuroscience. 1997;17:5528–5535. doi: 10.1523/JNEUROSCI.17-14-05528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Neuroscience. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Reiss Jones M, Moynihan H, MacKenzie N, Puente J. Temporal aspects of stimulus-driven attending in dynamic arrays. Psychological Science. 2002;13:313–319. doi: 10.1111/1467-9280.00458. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Structured clinical interview for DSM-III-R – Nonpatient edition (SCID-NP, Version 1.0) Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- Stanford L, Santi A. The dopamine D2 agonist quinpirole disrupts attention to temporal signals without selectively altering the speed of the internal clock. Psychobiology. 1998;26:258–266. [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanisms of conscious integration. Brain Research – Brain Research Reviews. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Monaco C, McMichael H, Tyson K, Chambliss C, Christensen HL, Celenza MA. Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Perceptual and Motor Skills. 1998;86:515–526. doi: 10.2466/pms.1998.86.2.515. [DOI] [PubMed] [Google Scholar]
- Tysk L. Time estimation by healthy subjects and schizophrenic patients: A methological study. Perceptual and Motor Skills. 1983a;56:983–988. doi: 10.2466/pms.1983.56.3.983. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time and the subclassification of schizophrenic disorders. Perceptual and Motor Skills. 1983b;57:911–918. doi: 10.2466/pms.1983.57.3.911. [DOI] [PubMed] [Google Scholar]
- Tysk L. Estimation of time by patients with positive and negative schizophrenia. Perceptual and Motor Skills. 1990;71:826. doi: 10.2466/pms.1990.71.3.826. [DOI] [PubMed] [Google Scholar]
- Volz H-P, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: An fMRI study at adjusted levels of difficulty. NeuroReport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Wahl OF, Sieg D. Time estimation among schizophrenics. Perception and Motor Skills. 1980;50:535–541. doi: 10.1177/003151258005000232. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Seidman LJ, Makris N, Tsuang MT, Kennedy DN, Goldstein JM. Neuroanatomic substrates of sex differences in language dysfunction in schizophrenia: A pilot study. Schizophrenia Research. 2007;90:295–301. doi: 10.1016/j.schres.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearden JH, Edwards H, Fakhri M, Percival A. Why sounds are judged longer than lights: Application of a model of the internal clock in humans. The Quarterly Journal of Experimental Psychology. 1998;51B:97–120. doi: 10.1080/713932672. [DOI] [PubMed] [Google Scholar]
- Zakay D, Block RA. The role of attention in time estimation processes. In: Pastor MA, Artieda J, editors. Time, internal clocks and movement. New York: Elsevier Sciences B.V.; 1996. pp. 143–164. [Google Scholar]


