Abstract
The treatment of acute and chronic pain is still deficient. The modulation of glial cells may provide novel targets to treat pain. We hypothesize that astrocytes and microglia participate in the initiation and maintenance of both, acute surgical and chronic neuropathic pain. Rats underwent paw incision, L5 nerve exposure or L5 nerve transection surgery. Behavioral mechanical allodynia was assessed using von Frey filaments. Immunohistochemistry was performed using anti-ionized calcium binding adaptor protein, Iba-1 (microglia), and anti-Glial Fibrillary Acidic Protein, GFAP (astrocytes) on day 1, 4 and 7 after surgery. Following paw incision and at spinal L5 segment GFAP expression was increased in laminae I-II and Iba1 in deep laminae on day 1, in the entire dorsal horn on day 4 and dissipate on day 7 after paw incision in parallel with the allodynia. L5 nerve transection induced mechanical allodynia from day 1 to 7 which correlated with Iba-1 increases on day 1, 4 (entire dorsal horn) and day 7 after nerve injury (deep laminae of the dorsal horn) at spinal L5 segment. Conversely, GFAP increased at later time points from day 4 (deep laminae) and on day 7 (entire dorsal horn). Our data demonstrates that astrocytes (GFAP expression) play a role in the initiation of acute pain and the maintenance of chronic pain while Iba-1 increases closely correlated with the early phase of neuropathic pain. Iba1 and GFAP increased rostrally, at L3 segment, after paw incision (day 4) and only Iba1 increased following L5 nerve transection (day 7).
Keywords: paw incision, neuropathic pain, postoperative pain, spinal cord, allodynia, glia
Introduction
Despite the considerable advancements in our understanding of the mechanisms of acute and chronic pain, inadequate pain relief remains a considerable issue in acute postoperative and chronic (pathologic) pain conditions. A vast percentage of patients (∼40%) receiving analgesics continue to experience moderate to extreme postoperative pain (Brennan et al., 2007; Dolin et al., 2002). Additionally, about 25% of patients receiving analgesic treatment after surgery show symptoms compatible with pain medication-related side-effects (Dolin and Cashman, 2005; Warfield and Kahn, 1995). Furthermore, acute postoperative pain may lead to persistent postsurgical pain (20−50%) and disability (5−10%) in common surgical procedures such as amputation, breast surgery, thoracotomy or coronary artery bypass surgery (Kehlet et al., 2006). In most patients, postsurgical chronic pain manifests similarly to neuropathic pain (Jung et al., 2003; Kehlet et al., 2006). In addition, pain affects 70% of the 10 million cancer patients diagnosed each year and 60−100% of patients suffering from HIV/AIDS will experience pain during their illness (Cousins et al., 2004). Neuropathic pain, resulting from nerve injury, is often the most difficult to treat. A new treatment strategy is necessary because the majority of analgesics possess modest effectiveness, induce physical dependence, produce significant side effects or are not effective in all types of pain (Dworkin et al., 2003). A better understanding of the mechanisms underlying acute and chronic pain will aid in the development of more effective and safer drugs for pain syndromes.
The recognition of the critical role of glia in the pathophysiological process in acute and chronic pain conditions opens a new and promising avenue to new pain treatment strategies (Scholz and Woolf, 2007). It is generally believed that glial cells contribute to hypersensitivity mainly in chronic pain conditions. However, spinal microglia and astrocytes play a major role in the development and maintenance of allodynia and hyperalgesia in acute, subacute and chronic pain states. Microglia and astrocytes react to peripheral insults such as paw incision (Obata et al., 2006; Romero-Sandoval and Eisenach, 2007), carrageenan-induced paw inflammation (Hua et al., 2005), complete Freunds adjuvant-induced paw inflammation (Raghavendra et al., 2004), complete Freunds adjuvant-induced monoarthritis (Sun et al., 2007), zymosan-induced peripheral nerve inflammation (Ledeboer et al., 2005) or peripheral nerve injury (Jin et al., 2003; Tanga et al., 2005; Tsuda et al., 2003). These studies suggest that different types of pain may share a common pathophysiological mechanims, and that this may lie in the spinal cord immune response.
A direct comparison of glial reactivity between acute vs. chronic rodent pain models has not been done thus far. The current study tests the hypothesis that microglia and astrocytes participate in the development and maintenance of both acute postoperative and chronic neuropathic pain states. We compared the L5 nerve transection chronic neuropathic pain model with the acute paw incision model. Our laboratory has extensively characterized the glial responses in the L5NT model. We have demonstrated that microglial reactivity is involved not only in the initiation (Raghavendra et al., 2003) but also in the long-term maintenance of L5NT-induced hypersensitivity (Tawfik et al., 2007), and that astrocytes are mainly reactive in later phases of behavioral hypersensitivity following L5NT (Raghavendra et al., 2003). The paw incision pain model induces acute hypersensitivity by at least day 1 and persists three-four days after surgery. This hypersensitivity spontaneously dissipates by day 7 after paw incision (Brennan et al., 1996). We and others have shown that paw incision induces microglial reactivity (Obata et al., 2006; Romero-Sandoval and Eisenach, 2007; Zhu et al., 2003).
Results
Paw incision- and L5 nerve transection -induced allodynia
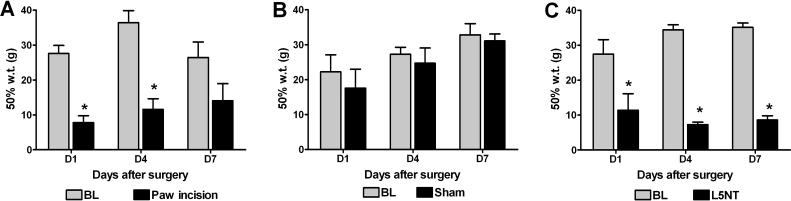
Paw incision induced mechanical allodynia on day 1 and 4 and by day 7 the mechanical withdrawal thresholds were not significantly different from baseline values (Figure 1A). Normal animals and the contralateral side to paw incision surgery showed similar mechanical withdrawal thresholds (data not shown). L5 spinal nerve exposure sham surgery did not alter mechanical withdrawal thresholds at any time points tested (Figure 1B). L5NT induced mechanical allodynia from day 1 to 7 (Figure 1C).
Figure 1.
50% withdrawal thresholds ipsilateral to paw incision (A), L5 exposure sham surgery (B) or L5 nerve transection (L5NT, C) before (base line = BL) and one, four and seven days (D1, D4 and D7 respectively) after surgery. *P<0.05 vs. base line value, t tests or Mann–Whitney U test when normality failed. N=4 for all groups.
Iba1 expression
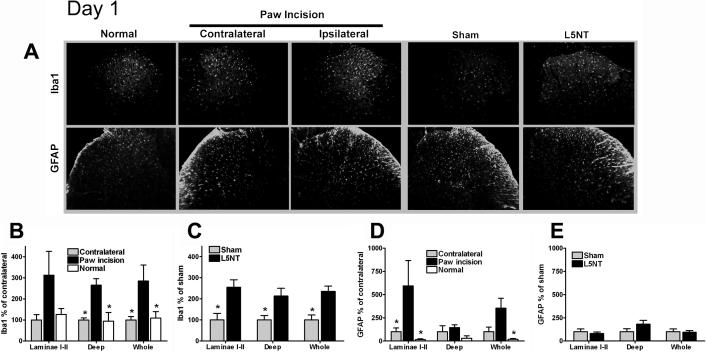
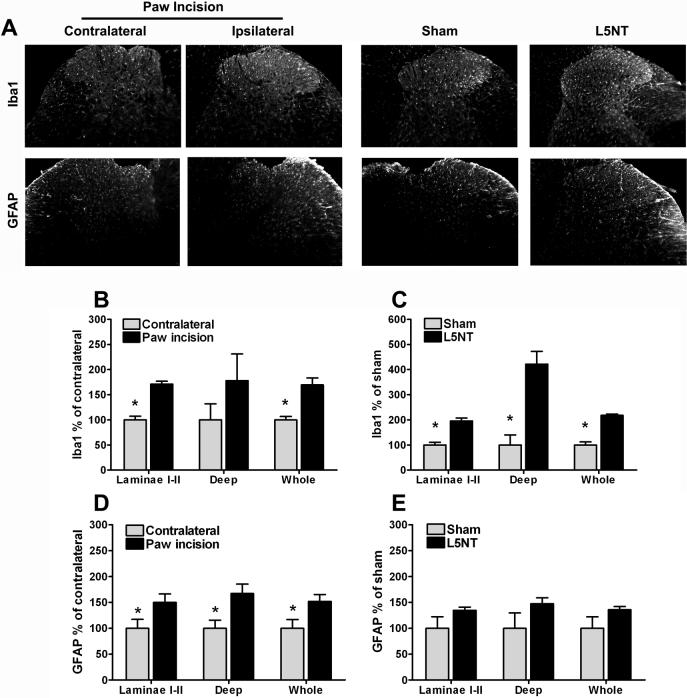
At day 1 post paw incision surgery, we observed a significant increase in Iba1 expression in the deep laminae (but not laminae I-II) of the dorsal horn compared to the dorsal horn contralateral to the incision or to normal naïve tissue (Figures A and B). One day after surgery, Iba1 expression was significantly increased throughout the dorsal horn (laminae I-II and deep laminae) in L5NT compared to sham surgery group (Figures 2A and C).
Figure 2.
Representative images and quantification of Iba1 and GFAP immunofluorescence staining at dorsal horn of L5 spinal cord. (A) L5 spinal cord sections of day 1 after surgery groups in normal naïve animals (column 1), contralateral (column 2) and ipsilateral (column 3) to paw incision, ipsilateral to sham surgery (column 4), and ipsilateral to L5 nerve transection (column 5). Quantification of normalized Iba1 and GFAP staining intensity on day 1 following paw incision surgery in normal, contralateral and ipsilateral to paw incision dorsal horn (B and C) and in L5 nerve transection (L5NT) and L5 nerve exposure sham surgery dorsal horn (D and E). The staining was quantified as pixels both at laminae I-II and at entire dorsal horn (whole). Deep dorsal horn (deep) data were calculated by subtracting the value of laminae I-II from the value of whole dorsal horn. Data were normalized using contralateral group (for paw incision model) or L5 nerve exposure sham group (for L5 nerve transection) values as 100%. *P<0.05 (t tests or Mann-Whitney U test when normality failed) vs. ipsilateral to paw incision group in B and D, and vs. L5 nerve transaction group in C.
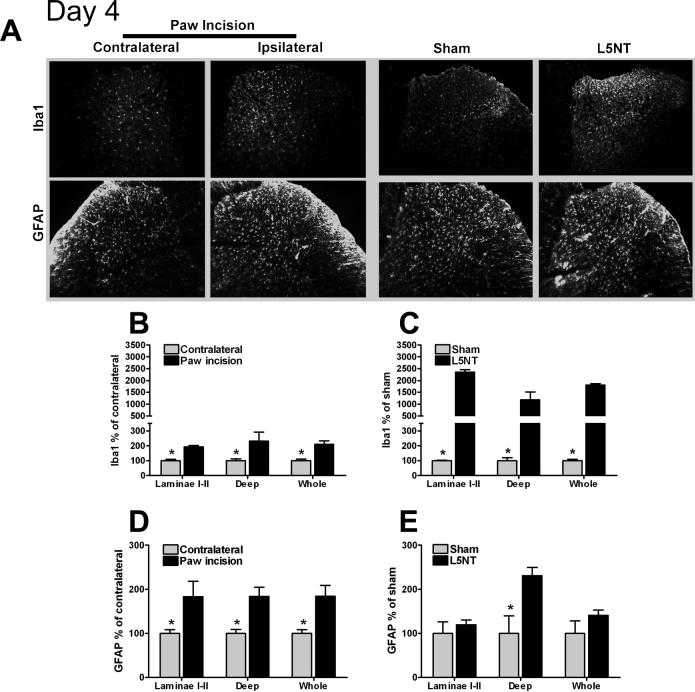
At day 4 after paw incision surgery, a significant increase in Iba1 expression was observed throughout the dorsal horn (laminae I-II and deep laminae) compared to contralateral to the incision side (Figures 3A and B). Four days after surgery, Iba1 expression continued to be significantly enhanced throughout the dorsal horn in L5NT compared to sham animals (Figures 2A and C).
Figure 3.
Representative images and quantification of Iba1 and GFAP immunofluorescence staining at dorsal horn of L5 spinal cord. (A) L5 spinal cord sections of day 4 after surgery groups in contralateral (column 1) and ipsilateral (column 2) to paw incision, ipsilateral to sham surgery (column 3) and ipsilateral to L5 nerve transection (column 4). Quantification of normalized Iba1 and GFAP staining intensity on day 4 following paw incision surgery in contralateral and ipsilateral to paw incision dorsal horn (B and D) and in L5 nerve transection (L5NT) and L5 nerve exposure sham surgery dorsal horn (C and E). The staining was quantified and normalized as described in Figure 2. *P<0.05 (t tests or Mann-Whitney U test when normality failed) vs. ipsilateral to paw incision group in B and D, and vs. L5 nerve transaction group in C and E.
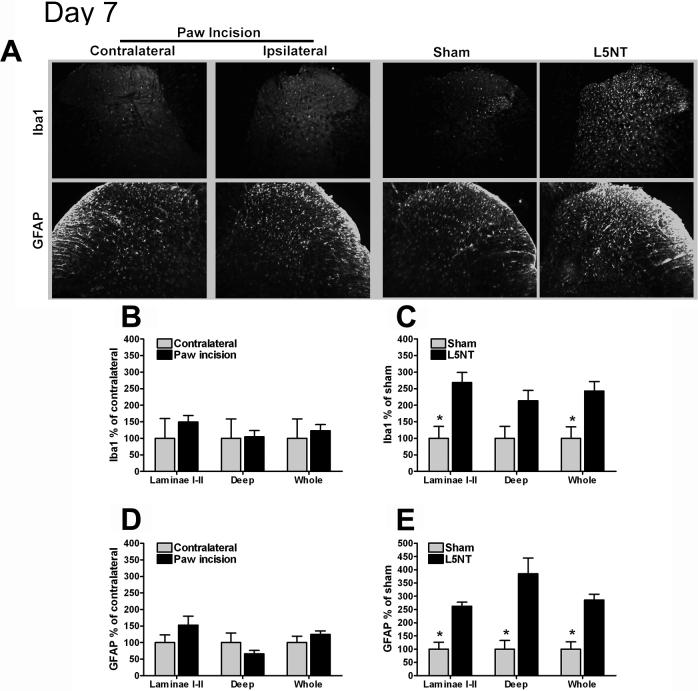
At day 7, Iba1 expression in both superficial and deep dorsal horn was not different between the ipsilateral and contralateral sides following paw incision surgery. This corresponded to the absence of allodynia on day 7 after surgery (Figures 4A and B). In the neuropathic pain model, Iba1 expression remained significantly higher in the laminae I-II of the dorsal horn in L5NT compared to sham surgery group. However, Iba1 expression was no longer different in L5NT and sham surgery groups in deep laminae at this time point (P=0.055, Figures 4A and B).
Figure 4.
Representative images and quantification of Iba1 and GFAP immunofluorescence staining at dorsal horn of L5 spinal cord. (A) L5 spinal cord sections of day 7 after surgery groups in contralateral (column 1) and ipsilateral (column 2) to paw incision, ipsilateral to sham surgery (column 3) and ipsilateral to L5 nerve transection (column 4). Quantification of normalized Iba1 and GFAP staining intensity on day 7 following paw incision surgery in contralateral and ipsilateral to paw incision dorsal horn (B and D) and in L5 nerve transection (L5NT) and L5 nerve exposure sham surgery dorsal horn (C and E). The staining was quantified and normalized as described in Figure 2. *P<0.05 (t tests or Mann-Whitney U test when normality failed) vs. L5 nerve transaction group in C and E.
In order to assess the glial phenotype at a rostral level, we study the Iba1 expression in spinal L3 segment at day 4 following paw incision and day 7 following L5 nerve transection (the time points where the Iba1 increase was more robust for each model). At day 4 after paw incision, Iba1 expression in laminae I-II of dorsal horn in L3 segment was significantly increased compared to contralateral to the incision side (Figures 5A and B). No significant differences were found in deep dorsal horn. Seven days after peripheral nerve injury, Iba1 expression was significantly enhanced throughout the dorsal horn compared to sham animals (Figures 5A and C).
Figure 5.
Representative images and quantification of Iba1 and GFAP immunofluorescence staining at dorsal horn of L3 spinal cord. (A) L3 spinal cord sections of day 4 in contralateral (column 1) and ipsilateral (column 2) to paw incision, and of day 7 in ipsilateral to sham surgery (column 3) and ipsilateral to L5 nerve transection (column 4). Quantification of normalized Iba1 and GFAP staining intensity on day 4 following paw incision surgery in contralateral and ipsilateral to paw incision dorsal horn (B and D) and on day 7 following L5 nerve transection (L5NT) and L5 nerve exposure sham surgery dorsal horn (C and E). The staining was quantified and normalized as described in Figure 2. *P<0.05 (t tests or Mann-Whitney U test when normality failed) vs. ipsilateral to paw incision group in B and D, and vs. L5 nerve transaction group in C.
Microglia in normal or contralateral to paw incision spinal dorsal horn were characterized by a small soma and highly ramified processes (non-reactive or surveilling microglia (Hanisch and Kettenmann, 2007)). A similar morphology was seen in spinal dorsal horn seven days after paw incision. However, in ipsilateral to L5NT (day 1−7 after surgery) or paw incision spinal dorsal horn (day 1 and 4 after surgery) the phenotype of microglia was characterized by cells with rounded cell bodies and thicker processes (reactive microglia (Hanisch and Kettenmann, 2007)).
GFAP expression
At day 1 after paw incision surgery, a significant increase in GFAP expression was observed in laminae I-II (but not in deeper laminae) compared to the dorsal horn contralateral to the incision (Figures 2A and D). One day after surgery, GFAP expression was similar in the dorsal horn in L5NT compared to sham surgery group (Figures 2A and E).
At day 4 after paw incision surgery, GFAP expression was significantly increased throughout the dorsal horn (laminae I-II and deep laminae) compared to the contralateral side following paw incision (Figures 3A and D). Four days after L5NT surgery, GFAP expression was increased in the deep laminae (but not in laminae I-II) compared to sham surgery group (Figures 3A and E).
At day 7, GFAP expression in both laminae I-II and deep laminae was not different between the ipsilateral and contralateral sides after paw incision surgery (Figures 4A and D). This corresponded to the return of withdrawal thresholds to baseline on day 7 after paw incision. In the neuropathic pain model, GFAP expression was increased throughout the dorsal horn in the L5NT group compared to the sham surgery group (Figures 4A and E).
In order to assess the glial phenotype at a rostral level, GFAP expression in spinal L3 segment at day 4 following paw incision and day 7 following L5 nerve transection (the time points where the GFAP increase was more robust for each model) was assessed. At day 4 after paw incision, GFAP expression in laminae I-II of dorsal horn in L3 segment was significantly increased compared to contralateral to the incision side (Figures 5A and B). No significant differences were found in deep dorsal horn. Seven days after peripheral nerve injury, Iba1 expression was significantly enhanced throughout the dorsal horn compared to sham animals (Figures 5A and C).
Discussion
The main observation of our study is that spinal glial reactivity is associated with both acute (paw incision) and chronic (L5 nerve transection) pain states. These glial changes have different anatomical and temporal patterns and are not confined to spinal L5 segment, but extend rostrally to the spinal L3 segment in the two rodent pain models. In agreement with our previous studies (Romero-Sandoval and Eisenach, 2007), we observed an increase in the expression of Iba1 (microglial marker) and GFAP (astrocytic marker) in the lumbar dorsal horn ipsilateral to paw incision. An enhanced expression of GFAP was observed in laminae I-II on day 1 and in the entire dorsal horn by day 4. Peripheral afferents are sensitized and spontaneously active after paw incision (Pogatzki et al., 2002), and it has been postulated that this increased input and subsequent sensitization of spinal neurons contribute to glial reactivity. The location of astrocytic reactivity suggests that astrocytes are responding to the primary afferent input to the spinal cord. Following paw incision spinal neuronal activity, as measured by c-fos expression, is increased in dorsal horn laminae I-II early following paw incision (Shimode et al., 2003).
Paw incision-induced spinal neuron sensitization is known to depend on central glutamatergic signaling through AMPA/kainate rather than NMDA receptors (Zahn et al., 2005). AMPA/kainite receptors are expressed at the astrocytic end feet in the central nervous system (Brand-Schieber et al., 2004) and glutamate and kainite evoke membrane currents in astrocytes located in laminae I-II in rat spinal cord slices during postnatal development (Ziak et al., 1998). AMPA evokes inward currents and stimulates ATP release in spinal cord astrocyte cultures, an effect that is potentiated by substance P (Werry et al., 2006). Interestingly, the blockade of the tachykinin NK1 receptor using a single dose of the specific antagonist, CP 154075, prevents (but not reverses) the development of hypersensitivity following paw incision and this effect lasted 72 hrs. These data together suggest, first, that AMPA/kainate and NK1 receptor stimulation in this model is a likely cause of early astrocytic reactivity; second, that this is dependent on the input from the injured site; and third, that astrocytic reactivity takes place in the development of the hypersensitivity induced by paw incision.
Our current results are in contrast with the generally accepted assumption that astrocytes are involved in the maintenance, but not in the development of pain, especially in chronic pain states. Supporting the idea that astrocytes are involved in the initiation of some types of pain, others have shown that astrocytes are responsible of the initiation of persistent orofacial pain (a trigeminal model of inflammation and persistent pain model), and that the astrocytic reactivity observed in this model is triggered by the neuronal input from the peripheral injured site and the neurotransmitter, substance P (Guo et al., 2007).
Microglia can be activated to produce pro-inflammatory cytokines through AMPA/kainate receptors (Noda et al., 2000), and to activate NF-kB by substance P (Rasley et al., 2002). Microglial reactivity was observed in deep dorsal horn laminae one day after paw incision surgery and in the entire dorsal horn later on (day 4). It is possible that early microglial reactivity in this model is due to the initial astrocytic reactivity. Other factors (that may be produced by astrocytes) such as ATP, cytokines, chemokines, nitric oxide or prostaglandins have been shown to participate in microglial activation (DeLeo et al., 2006). It is also possible that these factors contribute to the paw incision-induced microglial reactivity. Our current and previous results (Romero-Sandoval and Eisenach, 2007) show a better correlation between hypersensitivity and spinal Iba1 expression following paw incision surgery compared to previous studies using the microglial marker, OX-42 (Obata et al., 2006; Zhu et al., 2006). This suggests that Iba1, a cytosolic clacium biding protein expressed in microglia, is a better marker for microglial reactivity in this model.
Paw incision-induced GFAP and Iba1 expression was associated with the initiation (day 1) and maintenance (day 4) of the mechanical hypersensitivity. Importantly, microglial and astrocytic reactivity dissipated on day 7 after surgery, in accordance with the absence of hypersensitivity. These findings show the dynamic and plastic nature of glial cells in acute pain. Notably, we have shown that glial cells may be induced to a reactive phenotype (shown as a change in morphology and increased expression of glial markers) by a peripheral injury (paw incision), but also return to a non-reactive state (or surveillance state) at the same pace to the return of behavioral hypersensitivity to baseline levels. It would be an over simplification if we argue that our findings, based on this glial plasticity (glial reactivity and normalization), suggest that glial reactivity is only responsible for paw incision-induced hypersensitivity. It has been shown that microglia, for example, play important roles in homeostasis and may be neuroprotective or induce healing in several central nervous system processes (Hanisch and Kettenmann, 2007). Therefore, our study may reflect a beneficial role of glial cells in acute pain. However, when we combine our previous data as well as other results, it appears more likely that these reactive microglia play a deleterious role. For example, it has been shown that glial inhibition using fluorocitrate (Obata et al., 2006) or the cannabinoid receptor type 2 agonist, JWH015 (Romero-Sandoval and Eisenach, 2007), reverses paw incision-induced mechanical hypersensitivity in parallel with a reduction of spinal glial reactivity. In addition, other glial modulators induce antinociception in neuropathic pain models (Ledeboer et al., 2005; Romero-Sandoval et al., 2008; Sweitzer et al., 2001; Tanga et al., 2005; Tawfik et al., 2007). Our data show that glial reactivity is physiological reversible, suggesting that a certain degree of glial reactivity may block the sensitization of spinal nociceptive neurons, leading to a resolution of hypersensitivity, while an over-reactivity is detrimental and may promote central sensitization which is more difficult to treat.
Microglial reactivity is integral for the initiation of peripheral nerve injury-induced hypersensitivity (Raghavendra et al., 2003). But it is also involved in the long-term maintenance of neuropathic pain (Tawfik et al., 2007). In contrast with the findings in the paw incision pain model, we observed that following L5NT, first, Iba1 expression was increased at the entire dorsal horn one day after L5NT and this persisted until seven days, and second, no significant GFAP expression was observed at day 1, which was increased at day 4 and 7 following nerve injury. These findings are in accordance with the hypothesis that microglial cells are the first responders in the spinal cord following peripheral nerve injury. In accordance with the glial marker expression and hypersensitivity correlation in the paw incision model, both, Iba1 and GFAP over-expression parallel the initiation and maintenance of the hypersensitivity induced by L5NT from day 1 to day 7.
Several factors may induce microglial reactivity following peripheral nerve injury. Among the most plausible are TLR-4 (Tanga et al., 2005), ATP (Tsuda et al., 2003), matrix metalloprotease-9 and cytokines (Kawasaki et al., 2008). Fractalkine was postulated as a trigger for microglial reactivity (Milligan et al., 2005), but recent studies have shown that fractalkine in fact protects against microglial neurotoxicity (Cardona et al., 2006). Additionally, microglial reactivity may be triggered through AMPA/kainate receptors (Noda et al., 2000) and substance P (Rasley et al., 2002). The peripheral nerve injury-induced changes at the local injury site, including the increased afferent nerve activity and algesic mediator release such as nitric oxide, ATP, excitatory amino acids, neuropeptides, etc, may precipitate spinal glial changes (De Leo et al., 2006; Pogatzki et al., 2002; Scholz and Woolf, 2007; Watkins et al., 2001). It is possible that all these factors contribute to the microglial reactivity observed on day 1 after nerve injury in the dorsal horn.
Astrocytes appear to be largely responsible for the maintenance or chronicity of hypersensitivity following peripheral nerve injury (Kawasaki et al., 2008; Ledeboer et al., 2005; Raghavendra et al., 2003). Our current findings are in agreement with this. We have previously shown that GFAP protein expression did not change four days after L5NT in laminae I-II of the spinal cord dorsal horn (Romero-Sandoval and Eisenach, 2007). Herein, we confirm this and found out that a more accurate anatomical analysis in the dorsal horn on day 4 after peripheral nerve injury reveals an increase in GFAP expression in deeper, but not in laminae I-II. Our laboratory has also shown increases in GFAP mRNA four days after L5NT, suggesting that the spinal astrocytic reactivity is taking place in a transcriptional and post-transcriptional level at this time point (Tanga et al., 2004; Tanga et al., 2006).
It has been hypothesized that peripheral nerve injury-induced astrocytic reactivity occurs secondary to the microglial reactivity. The onset of GFAP over-expression on day 4 following nerve injury in our current study supports this hypothesis. It has recently been shown that matrix metalloprotease-2 or interleukin-1β increases spinal GFAP expression and induces a pro-inflammatory phenotype in astrocytes (induction of pERK expression and/or interleukin-1) in association with allodynia in models of chronic neuropathic or inflammatory pain (Guo et al., 2007; Kawasaki et al., 2008). The matrix metalloprotease-2 mechanism seems to be independent of the microglial reactivity, however interleukin-1β is produced in association with microglial reactivity and the matrix metalloprotease-9 (Kawasaki et al., 2008).
Beggs and Salter have shown that sciatic nerve injury induces an increase in spinal Iba1 that extends from L3 to L5 (Beggs and Salter, 2007). Herein, we show that L5 nerve transection and paw surgical incision induces an increase in Iba1 not only at L5, but also at the L3 segment. Interestingly, we did not observe significant changes in GFAP expression after L5 nerve transection at spinal L3, indicating that astrocytes react more locally following peripheral nerve injury. However, paw incision induced an increase in GFAP expression in the L3 segment. These observations demonstrate that both models share similar glial reactivity but possess different anatomical and temporal patterns of expression. The reactive microglia following both L5 nerve transection or surgical incision, and the reactive astrocytes following paw incision in L3 segment suggest that the function of second order neurons may be altered and may contribute to mechanical allodynia in postoperative and neuropathic pain. Whether the widespread glial reactivity in both models is due to cell migration, proliferation or due to peripheral or second order neurons excitability is plausible and intriguing.
Summary and conclusions
Our findings suggest the following: First, spinal astrocytes are the first cells to respond to peripheral surgical incision and both, spinal astrocytes and microglia show a reactive phenotype in the development and maintenance of acute surgical pain. Second, spinal microglia are the first responders to peripheral nerve injury and are reactive in the development of neuropathic pain, while spinal astrocytes show a reactive phenotype in the maintenance of nerve injury-induced hypersensitivity. However microglia certainly play a role in the long-term maintenance of neuropathic pain (Tawfik et al., 2007) . Third, based on previous pharmacological studies, glial reactivity in both, superficial or/and deep laminae of the dorsal horn positively regulates acute and chronic pain. These findings stress the importance of a detailed study of glial marker expression at different laminae of the spinal cord dorsal horn.
In conclusion, both, acute paw incision and L5 nerve transection pain models share glial reactivity as part of their pathophysiological response to injury. Since the resolution of acute pain parallels the spontaneous reduction of glial reactivity, this study leads to further investigation into the mechanism of action of this healing process, which may help in developing innovative strategies to treat chronic pain conditions.
Experimental procedure
Animals and surgeries
All procedures were conducted under approval by the Institutional Animal Care and Use Committee at Dartmouth College (Dartmouth Medical School, Hanover, New Hampshire) and in accordance with the Guidelines for Animal Experimentation of the International Association for the Study of Pain (IASP). Male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250−300 g at the start of surgery underwent L5 nerve transection (L5NT), L5 exposure sham or paw incision surgery as previously described (Brennan et al., 1996; Tanga et al., 2005). Briefly, for L5NT rats were anesthetized with 2% isoflurane in oxygen and a small incision to the skin overlying L5–S1 was made followed by retraction of the paravertebral musculature from the vertebral transverse processes. L4 and L5 spinal nerves were exposed by partially removing the L6 transverse process. The L5 spinal nerve was identified, lifted slightly, and transected. The wound was irrigated with saline and sutured in two layers. The sham surgery consisted of exposure of the L5 spinal nerve without transection.
For paw incision surgery, animals were anesthetized with 2% isoflurane in oxygen, and the plantar aspect of the left hind paw was prepared in a sterile manner with a 10% povidone–iodine solution. A midline incision (1 cm) from the heel to the base of the toes was performed using a No. 11 blade, using sterile technique. A small forceps was used to elevate the flexor tendon from the heel to the toes. The incision was closed using two inverted with 5.0 nylon mattress sutures. Animals were housed individually and maintained in a 12:12 hr light/dark cycle with ad libitum access to food and water. Efforts were made to limit animal distress and to use the minimum number of animals necessary to achieve statistical significance.
Behavioral testing
For behavioral testing calibrated von Frey filaments (Stoelting, Wood Dale, IL) were applied to the plantar aspect of the hind paws. The 50% withdrawal threshold to mechanical stimuli was measured twice at 10 min intervals ipsilaterally and contralaterally to surgery using an up–down statistical method (Chaplan et al., 1994) and the average of these values was used for data analysis. The withdrawal threshold was determined for each animal before surgery, 1, 4 and 7 days after surgery in independent groups.
Tissue preparation and Immunohistochemistry
To study glial reactivity we used antibodies against ionized calcium binding adaptor protein, Iba1 to label microglia, and anti-Glial Fibrillary Acidic Protein, GFAP to label astrocytes. Iba1 gene is located within the major histocompatibility complex class II region (human chromosome). Iba1 is involved in regulation of the actin cytoskeleton that contributes to motility and phagocytosis and in the signaling pathway of Rac, a molecule that regulates microglial migration, membrane ruffling, phagocytosis and radical formation (Imai and Kohsaka, 2002). GFAP is required for the normal myelination in the central nervous system (including spinal cord), the maintenance of the structural and functional integrity of the blood-brain barrier and the normal process elongation and motility of astrocytes (Liedtke et al., 1996). Messing et al. (Messing et al., 1998) has shown that transgenic mice that carry copies of the human GFAP gene possess hypertrophic astrocytes that up-regulate small heat-shock proteins (ubiquitin, aB-crystallin, and HSP25) and present eosinophilic aggregates. GFAP distribution in astrocytes labels process elongation and branching, which depends on nitric oxide and cyclic GMP formation (Boran and Garcia, 2007). Therefore, an over-expression of Iba1 or GFAP is interpreted as a sign of microglial or astrocytic reactivity and is associated with a pro-algesic phenotype, as shown in different pain models (Raghavendra et al., 2003; Romero-Sandoval and Eisenach, 2007).
Rats were euthanized on postoperative day 1, 4 and 7 (n=3−4 for all groups) to determine microglial or astrocytic reactivity induced by L5NT or paw incision. A L5 spinal nerve exposure was used as the sham surgery control group for L5NT. Normal naïve animals or the contralateral side to the surgery was used as control groups for paw incision group. Rats were deeply anesthetized with 2−4% isofluorane in oxygen and perfused transcardially with buffer (0.01M phosphate buffered saline, 150 ml) followed by 4% formaldehyde (350 ml) at room temperature. The L5 segment (and L3 segment for some groups) of the spinal cord was removed and placed in 30% sucrose for 48−72 hr at 4 °C. The tissue was then frozen at −80°C in O.C.T. Compound (Sakura Finetek, Torrance, CA). Immunohistochemistry and immunofluorescence were performed on transverse 20-μm L5 spinal cord free-floating sections. Spinal cord sections were washed (three times) in PBS, then were incubated in 3% FBS/PBS for 30 min room temperature. Primary antibodies were applied overnight at 4°C. A rabbit polyclonal antibody for Iba1 was used to label microglia (1:500, Wako Pure Chemical Industries, Richmond, VA). A rabbit polyclonal antibody for GFAP was used to label astrocytes (1:10,000, Dako Cytomation, Glostrup, Denmark). The following day tissue sections were washed and then visualized with the appropriate secondary fluorescent antibody, goat anti-rabbit IgG Alexa Fluor™-488 (1:250, 1 hr at room temperature, Molecular Probe, Invitrogen, Carlsbad, CA). Finally, tissue sections were washed and mounted with Vectashield (Vector Labs, Burlingame, CA) containing 4’,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI, Sigma, St Louis, MO) to visualize cell nuclei. Specificity of each assay was tested by omitting the primary antibody. The sections were examined with an Olympus fluorescence microscope, and images were captured with a Q-Fire cooled camera (Olympus, Melville, NY). The tissue used to study microglial and astrocytic activation was harvested from the same animals that were tested for behavioral data.
Glial reactivity has previously been determined by comparing immunofluorescence staining intensity (Romero-Sandoval and Eisenach, 2007; Romero-Sandoval et al., 2008). Herein, we quantified the Iba1 and GFAP staining, blinded to experimental conditions, as the number of pixels above a preset intensity threshold using SigmaScan Pro 5 as previously described (Romero-Sandoval and Eisenach, 2007; Romero-Sandoval et al., 2008). The Iba1 or GFAP staining intensity was examined in a standardized area of laminae I–II or of the entire dorsal horn with 3−4 slices examined per animal. Deep dorsal horn data were calculated by subtracting the value of laminae I-II from the value of whole dorsal horn. Glial reactivity is characterized by an increase in the number (proliferation), migration and the complexity of these cells (rounded cell bodies and thicker processes), resulting in an increase in labeling. Therefore, an increase in the number of pixels was interpreted as a sign of glial reactivity. Immunohistochemistry was performed in batches for each post-surgery day to decrease staining variability over time. Data are presented as the percent of control groups.
Statistical Analyses
Comparisons among groups and the effects of L5NT, L5 spinal nerve exposure only and paw incision surgery on withdrawal thresholds, microglial or astrocytic staining (in pixels) were performed using t tests or, when normality failed, Mann–Whitney U test. Data are presented as mean ± SEM. In all cases a P value less than 0.05 was considered significant. SigmaStat and GraphPad inStat software were used.
Acknowledgements
Supported in part by grant DA11276 from the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007;21:624–33. doi: 10.1016/j.bbi.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boran MS, Garcia A. The cyclic GMP-protein kinase G pathway regulates cytoskeleton dynamics and motility in astrocytes. J Neurochem. 2007;102:216–30. doi: 10.1111/j.1471-4159.2007.04464.x. [DOI] [PubMed] [Google Scholar]
- Brand-Schieber E, Lowery SL, Werner P. Select ionotropic glutamate AMPA/kainate receptors are expressed at the astrocyte-vessel interface. Brain Res. 2004;1007:178–82. doi: 10.1016/j.brainres.2003.12.051. [DOI] [PubMed] [Google Scholar]
- Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105:205–21. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cousins MJ, Brennan F, Carr DB. Pain relief: a universal human right. Pain. 2004;112:1–4. doi: 10.1016/j.pain.2004.09.002. [DOI] [PubMed] [Google Scholar]
- De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002;89:409–23. [PubMed] [Google Scholar]
- Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritis, and urinary retention. Evidence from published data. Br J Anaesth. 2005;95:584–91. doi: 10.1093/bja/aei227. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–18. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci. 2005;22:2431–40. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–74. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–22. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104:1–13. doi: 10.1016/s0304-3959(03)00241-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early-and late-phase development of neuropathic pain. Nat Med. 2008;14:331–6. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17:607–15. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- Messing A, Head MW, Galles K, Galbreath EJ, Goldman JE, Brenner M. Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am J Pathol. 1998;152:391–8. [PMC free article] [PubMed] [Google Scholar]
- Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22:2775–82. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–8. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Eisenach JC, Hussain H, Bynum T, Vincler M. Spinal glial activation contributes to postoperative mechanical hypersensitivity in the rat. J Pain. 2006;7:816–22. doi: 10.1016/j.jpain.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87:721–31. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–30. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–73. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Rasley A, Bost KL, Olson JK, Miller SD, Marriott I. Expression of functional NK-1 receptors in murine microglia. Glia. 2002;37:258–67. doi: 10.1002/glia.10034. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–94. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal Microglial and Perivascular Cell Cannabinoid Receptor Type 2 Activation Reduces Behavioral Hypersensitivity without Tolerance following Peripheral Nerve Injury. Anesthesiology. 2008 doi: 10.1097/ALN.0b013e318167af74. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Shimode N, Fukuoka T, Tanimoto M, Tashiro C, Tokunaga A, Noguchi K. The effects of dexmedetomidine and halothane on Fos expression in the spinal dorsal horn using a rat postoperative pain model. Neurosci Lett. 2003;343:45–8. doi: 10.1016/s0304-3940(03)00309-4. [DOI] [PubMed] [Google Scholar]
- Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Schubert P, DeLeo JA. Propentofylline, a glial modulating agent, exhibits antiallodynic properties in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2001;297:1210–7. [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–61. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, Nutile-McMenemy N, Marks A, Deleo JA. Role of astrocytic S100beta in behavioral hypersensitivity in rodent models of neuropathic pain. Neuroscience. 2006;140:1003–10. doi: 10.1016/j.neuroscience.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav Immun. 2007;21:238–46. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Warfield CA, Kahn CH. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology. 1995;83:1090–4. doi: 10.1097/00000542-199511000-00023. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–5. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Werry EL, Liu GJ, Bennett MR. Glutamate-stimulated ATP release from spinal cord astrocytes is potentiated by substance P. J Neurochem. 2006;99:924–36. doi: 10.1111/j.1471-4159.2006.04133.x. [DOI] [PubMed] [Google Scholar]
- Zahn PK, Pogatzki-Zahn EM, Brennan TJ. Spinal administration of MK-801 and NBQX demonstrates NMDA-independent dorsal horn sensitization in incisional pain. Pain. 2005;114:499–510. doi: 10.1016/j.pain.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Zhu X, Conklin D, Eisenach JC. Cyclooxygenase-1 in the spinal cord plays an important role in postoperative pain. Pain. 2003;104:15–23. doi: 10.1016/s0304-3959(02)00465-7. [DOI] [PubMed] [Google Scholar]
- Zhu X, Vincler MA, Parker R, Eisenach JC. Spinal cord dynorphin expression increases, but does not drive microglial prostaglandin production or mechanical hypersensitivity after incisional surgery in rats. Pain. 2006;125:43–52. doi: 10.1016/j.pain.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Ziak D, Chvatal A, Sykova E. Glutamate-, kainate- and NMDA-evoked membrane currents in identified glial cells in rat spinal cord slice. Physiol Res. 1998;47:365–75. [PubMed] [Google Scholar]