Abstract
The mammalian subventricular zone (SVZ) of the lateral wall of the forebrain ventricle retains a population of proliferating neuronal precursors throughout life. Neuronal precursors born in the postnatal and adult SVZ migrate to the olfactory bulb where they differentiate into interneurons. Here we tested the potential of mouse postnatal SVZ precursors in the environment of the embryonic brain: (i) a ubiquitous genetic marker, (ii) a neuron-specific transgene, and (iii) a lipophilic-dye were used to follow the fate of postnatal day 5–10 SVZ cells grafted into embryonic mouse brain ventricles at day 15 of gestation. Graft-derived cells were found at multiple levels of the neuraxis, including septum, thalamus, hypothalamus, and in large numbers in the midbrain inferior colliculus. We observed no integration into the cortex. Neuronal differentiation of graft derived cells was demonstrated by double-staining with neuron-specific β-tubulin antibodies, expression of the neuron-specific transgene, and the dendritic arbors revealed by the lipophilic dye. We conclude that postnatal SVZ cells can migrate through and differentiate into neurons within multiple embryonic brain regions other than the olfactory bulb.
The subventricular zone (SVZ) is a germinal layer that forms adjacent to the ventricular zone during embryonic development. Proliferation persists in the SVZ of the lateral ventricles throughout life in rodents (1–3), carnivores (4, 5), primates (6), and probably other mammals. In vitro, adult SVZ cells from mice and rats can give rise to neurons and glia (7, 8). The proliferating neuronal precursors form homotypic chains of cells that migrate tangentially into the olfactory bulb (OB) where they differentiate into local interneurons (9–12). Similar neuronal precursors may exist in humans (13).
The SVZ in the adult brain contains the largest pool of neuronal precursors and as such may have applications for neuronal replacement and brain repair (reviewed in refs. 14–16). However, neuronal precursors born in the SVZ have not been observed to migrate into and differentiate within non-OB central nervous system (CNS) locations (9–11). In addition, transplants of SVZ cells into various adult brain regions outside of the SVZ have not shown migration of cells from the graft site and incorporation into the brain parenchyma (10, 11), although local migration and incorporation occurs with neonatal SVZ cells that are grafted into neonatal striatum (17). Aside from the possibility that postnatal SVZ cells may have a restricted differentiation potential, this limitation of migration and differentiation may be caused by a lack of proper cues or the presence of inhibitory signals outside the olfactory bulb in the adult brain.
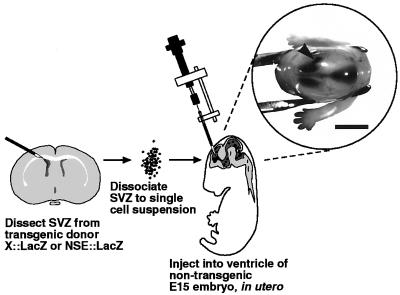
To test the ability of SVZ cells to interpret signals in the embryonic brain, we injected dissociated SVZ cells from postnatal animals into the developing cerebral ventricles (Fig. 1). Here we show that grafted SVZ cells can become incorporated into the embryonic hindbrain, midbrain, and forebrain and differentiate into neurons.
Figure 1.
Schematic of transplantation procedure of postnatal SVZ cells into the embryonic day (E) 15 forebrain ventricle. To illustrate that these injections target the embryonic ventricles, one recipient embryo was removed from the uterus after injection and is shown held at the neck by forceps in the Inset. Note that all ventricles were filled by the trypan blue dye which appears dark under reflected light. The arrowhead indicates the injection point. (Bar = 0.25 cm.)
METHODS
In Utero Transplantation.
SVZ (and striatal control) explants were dissected from coronal slices of P5–P10 (P, postnatal day) donor brains as described (7, 11). Explants were incubated 30 min at 37°C with gentle agitation in 0.06 μg/ml papain (Worthington) in 120 mM NaCl, 5 mM KCl, 25 mM glucose, and 20 mM Pipes (pH 7.4). Explants were then triturated with a fire-polished Pasteur pipet to a single cell suspension in DMEM (GIBCO) with 1 mg/ml DNase (Worthington). Cells were >99% viable by trypan blue exclusion. For PKH26 (Sigma) dye labeling of donor cells, the cells were incubated with diluted dye (5:1,000 in Diluent C) for 5 min at room temperature; the labeling reaction was stopped with 50% serum, and cells were washed three times with PBS. SVZ cells were resuspended for injection in DMEM at 50,000 cells/μl. As a control, SVZ cells were killed by four freeze–thaw cycles before grafting.
CD-1 mice timed-pregnant (E0 = vaginal plug date; P0 = date of birth) to E15 (Charles River Breeding Laboratory) were anesthetized with Metofane vapor and Nembutal (35 mg/kg body weight). A total of 125–250 μl of a 2.5% MgSO4 solution was given intraperitoneally as a smooth muscle relaxant. After a midline laparotomy, the uterine horns were exposed, and each embryo was manipulated in the uterus so that the forebrain ventricles were evident by transillumination. Approximately 1 μl of the cell suspension was injected into the ventricles. Trypan blue (0.1%) was sometimes added to the cell suspension to confirm targeting of injections. The freehand injections were performed with a glass capillary pipet (100–150 μm outer diameter with beveled tip) driven by a modified Narishige hydraulic micromanipulator. After all embryos were injected, the uterus was placed back into the abdominal cavity, and the mother was sutured and returned to a warmed cage. Approximately half of the operated mothers gave birth to pups that lived to at least P8. This experiment was performed in accordance with institutional guidelines.
Detection of Grafted Cells.
Between P1 and P36, recipient pups were deeply anesthetized with Nembutal and transcardially perfused with 0.1 M phosphate buffered (pH 7.4) 3% paraformaldehyde. For quantitation of 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal+) cellular incorporation, brains were first cryoprotected with 30% sucrose in 0.1 M phosphate buffer, then serially sectioned horizontally on a freezing sliding microtome at 80–100 μm. X-Gal histochemistry was performed as described (11). All sections were mounted and serially screened at ×200 on an Olympus inverted microscope. For detection of PKH26 cells, brains were postfixed overnight in the above fixative and Vibratome sectioned horizontally at 100 μm. PKH26 cells were imaged with a Zeiss Axiovert microscope fitted with a Princeton Instruments cooled charged-coupled device (CCD) camera by using a KAF 1400 chip with fluorescent (550 nm excitation) illumination.
Immunohistochemistry.
For TuJ1 staining, 80-μm frozen sections were blocked in 10% horse serum/PBS/0.3% Triton X-100 for 1 hr, incubated in 1:1,000 anti-Tuj1 mAb (kind gift of A. Frankfurter, University of Virginia, Charlottesville) overnight at 4°C, washed three times, and incubated for 3 hr at room temperature with a Cy3 secondary antibody (Sigma). For glutamic acid decarboxylase (GAD) staining, 100-μm Vibratome sections were processed floating, blocked as above, incubated with anti-GAD antibody (kind gift of I. Kopin, National Institutes of Health) at 1:1,000 at 4°C for 2 days, washed three times, incubated with a biotinylated secondary antibody (Vector Laboratories) for 3 hr at room temperature, and developed with the Vector Elite peroxidase kit (Vector Laboratories) by using 0.02% diaminobenzidine with 0.01% H2O2 in PBS. Tyrosine hydroxylase (TH) staining was performed as for GAD with 1:1,000 anti-TH antibody (Pel-Freez Biologicals).
Controls in which primary antibodies were omitted or replaced with pre-immune serum (for GAD) resulted in no detectable staining for both fluorescent and diaminobenzidine protocols.
RESULTS
Postnatal SVZ Cells Are Incorporated at Multiple Levels of the Developing Neuraxis.
To identify grafted cells we used transgenic X∷LacZ (18) males as the SVZ donors. X∷LacZ mice express β-galactosidase in all cells; the β-galactosidase product is localized to the nucleus. Nontransgenic mouse embryos at developmental day 15 (E15) received in utero an injection of 50,000 dissociated SVZ cells into the forebrain ventricle. Mice were born 4–5 days later and killed 12 days after birth.
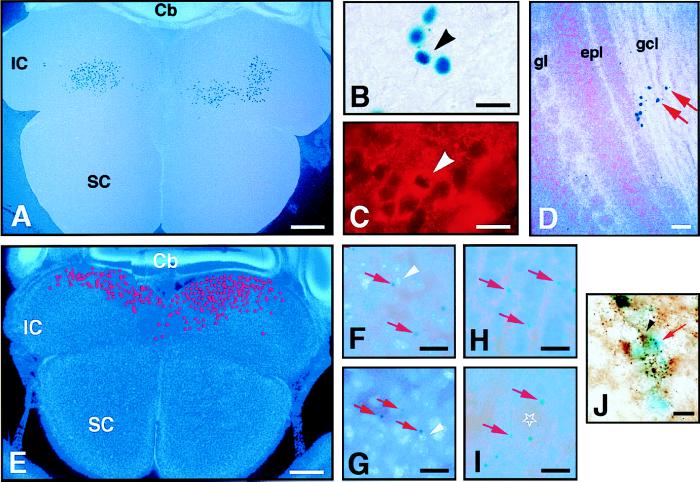
Graft-derived cells were not seen in the cortex or hippocampus, but could be found in the OB, septum, thalamus, hypothalamus, and prominently in some midbrain regions of the same animal (see Table 1). In all animals studied, the midbrain inferior colliculi (IC) had large numbers of graft-derived SVZ cells (Fig. 2 A and B). It is unlikely that this IC incorporation was caused by a misplaced injection into the midbrain parenchyma, as cells were found bilaterally, and graft-derived cells were also found in other CNS regions including the OB (Fig. 2D, Table 1).
Table 1.
Sites of incorporation of grafted SVZ cells
| Mother no.-mouse no. (age) | OB | Ctx | St | Hp | Sep | Th | HTh | SC | IC | Md | Cb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| X::LacZ SVZ transplants | |||||||||||
| 3-1 (P12) | ND | ND | ND | ND | ND | ND | ND | − | +++ | ND | ND |
| 3-2 (P12) | +++ | − | − | − | +++ | +++ | ++ | − | ++++ | +++ | − |
| (gcl) | |||||||||||
| 3-3 (P12) | − | − | − | − | − | ++ | +++ | − | +++ | ++ | + |
| (MB, LH) | |||||||||||
| NSE::LacZ SVZ transplants | |||||||||||
| 9-1 (P1) | + | ND | ND | ND | ND | + | ++ | ND | +++ | ND | ND |
| (gcl) | |||||||||||
| 1-2 (P8) | ++ | − | − | − | ++ | + | +++ | − | +++ | − | − |
| (gcl) | (MB) | ||||||||||
| 2-2 (P9) | ++ | − | − | − | − | − | − | ++++ | ++ | − | − |
| (gcl) | |||||||||||
| 2-5 (P11) | ++ | − | − | − | − | − | +++ | − | ++++ | − | − |
| (gcl) | (MB) | ||||||||||
| 2-6 (P11) | ++ | − | − | − | ++ | ++++ | +++ | − | ++++ | − | ++ |
| (gcl) | |||||||||||
| 4-3 (P11) | +++ | − | ND | ND | ND | ND | ND | − | ++++ | − | ND |
| (gl, gcl) | |||||||||||
| 4-4 (P11) | ++ | − | − | − | − | ++ | + | − | ++++ | − | ++ |
| (gcl) | (MB) | ||||||||||
| 1-7 (P12) | + | − | − | − | − | − | − | + | + | − | − |
| (gcl) | |||||||||||
| 1-6 (P12) | ++ | − | − | − | − | + | − | − | − | − | − |
| (gcl) | |||||||||||
| 1-5 (P12) | +++ | − | − | − | +++ | +++ | +++ | − | ++++ | − | +++ |
| (gl, gcl) | (MB, VM) | ||||||||||
| 4-5 (P36) | + | − | − | − | − | − | − | − | − | − | + |
| (gcl) | |||||||||||
| 4-6 (P36) | ++ | − | − | − | − | − | − | − | + | − | − |
| (gcl) | |||||||||||
| 4-7 (P36) | ++++ | − | ++ | − | − | + | + | − | ++ | − | − |
| (gl, gcl) | |||||||||||
| 4-8 (P36) | ++ | − | − | − | − | ++ | − | − | ++ | − | − |
| (gcl) | |||||||||||
| 4-9 (P36) | + | − | − | − | − | − | ++ | − | − | − | − |
| (gl) | (VM) |
Distribution of β-galactosidase positive cellular incorporation after transplantation of LacZ marked SVZ cells to the forebrain ventricle of E15 embryos. Brains were examined as horizontal sections. ++++, >500 positive cells; +++, >100 positive cells; ++, >10 positive cells; +, 1-10 positive cells; −, no detectable staining; ND, not determined. OB, olfactory bulb; Ctx, cortex; St, striatum; Hp, hippocampus; Sep, septum; Th, thalamus; HTh, hypothalamus; SC, superior colliculus; IC, inferior colliculus; Md, medulla; Cb, cerebellum; gcl, granule cell layer; gl, periglomerular cell layer; MB, mamillary body; LH, lateral hypothalamic nucleus; VM, ventromedial hypothalamic nucleus.
Figure 2.
(A–D) Transplant of postnatal X∷LacZ SVZ cells into the ventricle of E15 embryos. The recipient mouse was killed at P12 and the brain sectioned horizontally (animal 3-2, Table 1). X-Gal histochemistry produces a dark blue nuclear precipitate in X∷LacZ cells. In A and D, cell nuclei are counterstained with Hoechst 33258 and appear light blue under fluorescent illumination. (A) X-Gal+ cells in the IC. 490 cells were mapped in this 80-μm-thick section. (B) High-power view of the X-Gal+ cells in the inferior colliculus. (C) Example of X-Gal+ cell that was double-stained for the neuronal marker TuJ1 (arrowheads in B and C). (D) X-Gal+ cells in the granule layer of the olfactory bulb. (E–I) Transplant of postnatal NSE∷LacZ SVZ cells into the E15 ventricle. The recipient mouse was killed at P12 and the brain sectioned horizontally (animal 1-5, Table 1). An X-Gal perinuclear precipitate (typical examples indicated by red arrows) is produced in grafted cells which differentiate into neurons. All sections are counterstained with Hoechst 33258. (E) In this 80-μm-thick section, 790 X-Gal+ neurons were incorporated in the IC. Their mapped distribution is indicated by the red dots. (F) High-power view of E showing the graft-derived neurons in the IC. White arrowhead indicates nucleus corresponding to X-Gal positive cell. (G) Graft-derived neurons in the hypothalamus. (H and I) Graft-derived neurons in the olfactory bulb were found in the granule cell layer (H) and around glomeruli (star) (I). (J) Two GAD+/NSE∷LacZ+ neurons in the IC. The red arrow indicates the blue X-Gal deposit that became diffuse after the immunohistochemistry for GAD. The black arrows indicate a GAD+ cell body. Recipient killed at P1 (animal 9-1, Table 1). The localization of graft-derived cells in the IC varied from animal to animal (e.g., cells in A are more rostral, and in E more caudal). SC, superior colliculus; Cb, cerebellum; gl, glomerular layer; gcl, granule cell layer; epl, external plexiform layer. (Bars: A and E = 400 μm; D = 40 μm; B, C, F, G–I = 20 μm; J = 10 μm.)
Grafted SVZ Cells Differentiate into Neurons.
We double-labeled sections from animals receiving the SVZ carrying the X∷LacZ transgene with antibodies that recognize the neuron-specific type III β-tubulin (19). Many of the graft-derived cells were double-stained for this marker (Fig. 2 B and C). There were also graft-derived cells that did not stain with this antibody. With this method, however, we could only stain cells whose cell body was close to the surface of the section. Nonstained cells could correspond to glial cells or be too deep for antibody detection. To further show that transplanted cells can differentiate into neurons, we transplanted SVZ cells dissociated from postnatal NSE∷LacZ transgenic mice (20). These mice harbor a transgene carrying the LacZ reporter gene driven by the neuron-specific enolase (NSE) promoter. Differentiated neurons express the NSE∷LacZ transgene that can be detected as a small perinuclear deposit of blue X-Gal; neuronal precursors in the SVZ and glial cells do not express the transgene. Again, X-Gal+ neurons were detected at multiple levels of the neuraxis after transplantation, but not in the cortex or hippocampus (see Table 1). Recipients killed at approximately 2 weeks posttransplant often had many graft-derived neurons in the midbrain IC (Fig. 2 E and F). Graft-derived cells were also observed in the septum, thalamus, cerebellum, and hypothalamus (Fig. 2G, Table 1). X-Gal+ neurons were always detected in the OB (Fig. 2 H and I, Table 1). In recipients killed at 40 days posttransplant, NSE∷LacZ neurons were detected in multiple brain regions (see Table 1), demonstrating that some graft-derived neurons in non-OB locations survive for at least 40 days after transplantation. However, we notice that at P36, the longest survival studied, fewer cells were found outside the OB.
Control cells dissociated from NSE∷LacZ striatum underlying the SVZ did not migrate and differentiate within the embryonic brain, although diffusely blue cellular clumps were sometimes seen in the ventricles (n = 5, analyzed at P10). X-Gal+ cells were not seen when the NSE∷LacZ cells were killed before transplantation (n = 5, analyzed at P11).
To further characterize the phenotype of graft-derived neurons in the IC, we stained frontal section of animals that had received NSE∷LacZ SVZ grafts with TH and GAD antibodies. We found no evidence of TH positive cells, but a subpopulation of the cells expressing the transgene in the IC were also positive for GAD (Fig. 2J).
Vital Dye Labeling Reveals Dendritic Arbors on the Grafted Cells.
To reveal the morphology of the graft-derived cells, we labeled dissociated SVZ cells with the fluorescent lipophilic dye PKH26 before transplantation. The distribution of incorporated PKH26 labeled cells was essentially the same as observed with the NSE∷LacZ transgenic marker in two animals analyzed at P1, one analyzed at P3, and three analyzed at P11.
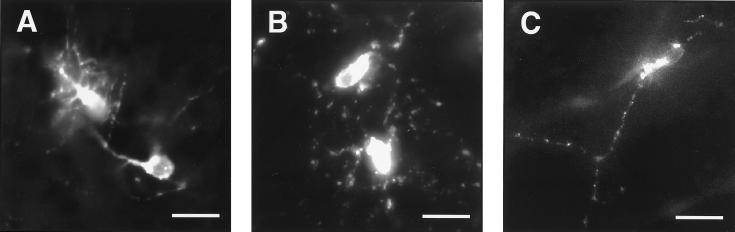
In the rostral migratory stream (RMS) between the SVZ and OB, graft-derived cells possessed the typical migrating cell morphology (9, 10, 21) (data not shown). In the OB granule cell layer, PKH26 labeled cells had morphologies similar to the resident granule neurons (Fig. 3C). This finding suggests that some graft-derived cells became incorporated into the SVZ and, like the resident SVZ neuronal precursors, migrated along the RMS and differentiated in the OB.
Figure 3.
Morphology of PKH26-labeled postnatal SVZ cells transplanted into the ventricle of E15 embryos. (A) Two neurons in the IC. Animal was killed at P11. (B) Two neurons in the hypothalamus. Animal was killed at P1. (C) Neuron in the granule layer of the OB. Animal was killed at P3. (Bars = 20 μm.)
PKH26 also revealed the morphology of cells that became incorporated in non-OB brain regions. Fig. 3A shows two labeled neurons in the IC; these cells were highly branched with many processes emanating from both the cell body and primary projection. This extensive arborization, not seen in OB interneurons (10, 21), resembles those of small stellate mouse IC neurons (22), suggesting that graft-derived cells can attain a neuronal structure in accordance with the location where they differentiate. Fig. 3B shows the stellate morphology of graft-derived cells in the hypothalamus.
No PKH26 labeled cells were observed in recipient brains when the labeled cells were killed before transplantation, though fluorescent debris could be found in the ventricles (n = 6, analyzed at P11). This control suggests that dye transfer does not contribute to the observed population of PKH26-labeled cells.
DISCUSSION
We used three markers to follow the fate of postnatal SVZ cells grafted into the embryonic ventricle. All three markers showed a similar pattern of SVZ cell integration at multiple levels of the neuraxis. The X∷LacZ marked cells revealed the wide range of CNS locations that SVZ cells can colonize, and many of these X∷LacZ cells were immunopositive for a neuron-specific β-tubulin. NSE∷LacZ transgenic cells demonstrated the same distribution of incorporation while also establishing that these grafted cells can differentiate into mature neurons: NSE is expressed at the time of synaptogenesis (23, 24), and both NSE and the NSE∷LacZ transgene are expressed only in postmitotic, postmigratory neurons (20); therefore, NSE∷LacZ in grafted SVZ cells suggests that they are mature neurons. This result was further substantiated by the morphology and complex dendritic arbors revealed by the PKH26 labeling.
The embryonic sites where SVZ cells became incorporated may provide cues for their migration and differentiation. As the brain matures, such signals may diminish except in regions where neuron addition persists; thus, postnatal SVZ cells may be chemically and spatially restricted from incorporation into regions of the brain where neuronal addition does not persist. Spatial segregation of environmental cues has been previously discussed: hippocampal neuronal precursor cells amplified in culture with basic fibroblast growth factor can differentiate in the neurogenic adult hippocampus and SVZ-OB system, but not within the nonneurogenic adult cerebellum (25).
The massive IC incorporation is particularly striking. The developing IC at the time of transplant (E15) may present robust incorporation and differentiation cues to which SVZ cells can respond. Alternatively, grafted cells in the ventricle may follow the natural cerebral spinal fluid flow and accumulate at the IC ventricular wall located at the narrow foramen between the third and fourth ventricle. Such high local concentrations of grafted cells may enhance heterotopic SVZ cell incorporation. If similarly high concentrations of grafted precursors could be attained in other walls of the developing ventricular system, massive incorporation, such as that observed in the IC, may occur in other brain regions. It is also possible that if SVZ grafts were performed earlier in embryonic development, the distribution of graft-derived cells would be more extensive or different.
Previous transplantation experiments suggest that CNS precursors have a wider potential than their normal pattern of differentiation would indicate (25–29). Intraventricular transplantation studies (refs. 27–29, and G.J.F., data not shown) have shown that embryonic neuronal precursors, irrespective of their origin, can migrate into essentially all regions of the embryonic brain. Our work demonstrates that postnatal SVZ neuronal precursors can also migrate into and differentiate within multiple CNS locations. However, unlike transplanted embryonic neuronal precursors, incorporation into some CNS targets (e.g., cortex, hippocampus; see Table 1) was not observed with SVZ grafts. This finding suggests that unlike embryonic precursors, SVZ cells are restricted in their incorporation or differentiation potential. In addition, large projection neurons were never found labeled in the PKH26-labeling experiments, consistent with the hypothesis that SVZ cells can only give rise to interneurons and not to large projection neurons. Some graft-derived cells expressed GAD, suggesting that these cells may be inhibitory interneurons. Compared with the postnatal SVZ, the embryonic neuronal precursor pools may contain a greater precursor diversity, and/or each precursor within those pools may have a greater potential.
Some of the graft-derived neurons in the IC assumed morphologies typical of resident neurons. Thus it appears that SVZ neuronal precursors are plastic in their final morphology. Many SVZ-derived neurons typically become GABAergic or dopaminergic in the OB (30), and the IC contains a large population of small (<15 μm diameter) GABAergic neurons (31). The size and morphology of some of the graft-derived neurons is similar to one class of GABAergic IC neurons, and some of the SVZ cells that became incorporated into this region expressed a GABAergic marker.
Spatial (32–35) and temporal (34) constraints are important in patterning brain development. This work reveals the wide range of CNS locations that postnatally derived neuronal precursors can colonize when spatial and temporal “boundaries” are experimentally crossed by transplantation. Therefore, our experiments not only reveal the developmental potential of the donor cells, but also demonstrate that various host regions throughout the developing brain can support the migration and differentiation of SVZ cells derived from the lateral wall of the lateral ventricle. This finding is consistent with the hypothesis that local cues play an important role in cell differentiation during brain histogenesis (26, 29, 36, 37).
Interesting to note, there was a trend of fewer graft-derived neurons outside the OB at 40 days posttransplant compared with the shorter survivals. Normal developmental cell death (38, 39) may account for the decreased numbers of transplanted neurons found in the midbrain. Although survival factors necessary for SVZ-derived neurons are maintained in adult OB, such factors may be present outside of the OB only during development.
Intercellular signals in the OB permit the continued migration, integration, and differentiation of SVZ cells throughout life. Remarkably, postnatal SVZ neuronal precursors can migrate into and differentiate within non-OB CNS regions when given access to multiple levels of the embryonic neuraxis. Thus, it appears that SVZ neuronal precursors are not cell-autonomously restricted from incorporation and differentiation within non-OB CNS locations. Regions outside the SVZ-OB system in the adult CNS may lack the proper signals for migration, differentiation, and survival of SVZ cells. Thus, with the provision of the proper cues at the desired target and an understanding of the range of phenotypes that these cells can generate, it may be possible to utilize the SVZ neuronal precursors for therapeutic grafting.
Acknowledgments
We thank S.-S. Tan for providing the X∷LacZ transgenic mice, S. Forss-Petters and P. Danielson for the NSE∷LacZ transgenic mice, Dr. I. J. Kopin (National Institutes of Health) for the anti-GAD antibody, and Dr. A. Frankfurter (University of Virginia, Charlottesville) for the anti-TuJ1 antibody. This work was supported by National Institutes of Health Grants R01 HD32116 and NS28478 (to A.A.-B.) and R01 NS32993 (to G.J.F.) D.A.L. is a National Institutes of Health Medical Scientist Training Program fellow supported by Grant 5T32GM07739. Preliminary work on these experiments was done by Christine Neyt. The schematic in Fig. 1 was based on one provided by D. Cooper.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: NSE, neuron-specific enolase; SVZ, subventricular zone; OB, olfactory bulb; IC, inferior colliculus; CNS, central nervous system; E, embryonic day; P, postnatal day; X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactoside; TH, tyrosine hydroxylase; GAD, glutamic acid decarboxylase.
References
- 1.Allen E. J Comp Neurol. 1912;22:547–568. [Google Scholar]
- 2.Privat A, Leblond C P. J Comp Neurol. 1972;146:277–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- 3.Smart I. J Comp Neurol. 1961;116:325–348. [Google Scholar]
- 4.Altman J. Anat Rec. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore W F, Jolly D R. J Neurocytol. 1972;1:69–84. doi: 10.1007/BF01098647. [DOI] [PubMed] [Google Scholar]
- 6.McDermott K W G, Lantos P L. Dev Brain Res. 1990;57:269–277. doi: 10.1016/0165-3806(90)90053-2. [DOI] [PubMed] [Google Scholar]
- 7.Lois C, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirschenbaum B, Goldman S A. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 10.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 11.Doetsch F, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankovski A, Sotelo C. J Comp Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser R A R, Goldman S A. Cereb Cortex. 1994;6:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Buylla A, Lois C. Stem Cells. 1995;13:263–272. doi: 10.1002/stem.5530130307. [DOI] [PubMed] [Google Scholar]
- 15.Gage F H, Ray J, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 16.Weiss S, Reynolds B A, Vescovi A L, Morshead C, Craig C G, Van der Kooy D. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 17.Bertarbet R, Zigova T, Bakay R A, Luskin M B. Cell Transplant. 1996;5:165–178. doi: 10.1177/096368979600500207. [DOI] [PubMed] [Google Scholar]
- 18.Tan S-S, Breen S. Nature (London) 1993;362:638–639. doi: 10.1038/362638a0. [DOI] [PubMed] [Google Scholar]
- 19.Easter S S, Ross L S, Frankfurter A. J Neurosci. 1993;13:285–299. doi: 10.1523/JNEUROSCI.13-01-00285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forss-Petter S, Danielson P E, Catsicas S, Battenberg E, Price J, Nerenberg M, Sutcliffe J G. Neuron. 1990;5:187–197. doi: 10.1016/0896-6273(90)90308-3. [DOI] [PubMed] [Google Scholar]
- 21.Kishi K. J Comp Neurol. 1987;258:112–124. doi: 10.1002/cne.902580109. [DOI] [PubMed] [Google Scholar]
- 22.Ramón y Cajal S. Histologie du Système Nerveux de l’Homme et des Vertébrés. Paris: Maloine; 1911. [Google Scholar]
- 23.Schmechel D E, Brightman M W, Marangos P J. Brain Res. 1980;190:195–214. doi: 10.1016/0006-8993(80)91169-5. [DOI] [PubMed] [Google Scholar]
- 24.Marangos P J, Schmechel D E, Parma A M, Goodwin F K. Brain Res. 1980;190:185–193. doi: 10.1016/0006-8993(80)91168-3. [DOI] [PubMed] [Google Scholar]
- 25.Suhonen J O, Peterson D A, Ray J, Gage F H. Nature (London) 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 26.Vicario-Abejón C, Cunningham M G, McKay R D G. J Neurosci. 1995;15:6351–6363. doi: 10.1523/JNEUROSCI.15-10-06351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell K, Olsson M, Björklund A. Neuron. 1995;15:1259–1273. doi: 10.1016/0896-6273(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 28.Rangel S, Leon M. Dev Brain Res. 1995;85:187–191. doi: 10.1016/0165-3806(94)00211-h. [DOI] [PubMed] [Google Scholar]
- 29.Fishell G. Development (Cambridge, UK) 1995;121:803–812. doi: 10.1242/dev.121.3.803. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-Buylla A. Semin Cell Dev Biol. 1997;8:207–213. doi: 10.1006/scdb.1996.0134. [DOI] [PubMed] [Google Scholar]
- 31.Roberts R C, Ribak C E, Oertel W H. Brain Res. 1985;361:324–338. doi: 10.1016/0006-8993(85)91303-4. [DOI] [PubMed] [Google Scholar]
- 32.Lumsden A. Trends Neurosci. 1990;13:329–335. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- 33.Puelles L, Rubenstein J L R. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- 34.McConnell S K. J Neurosci. 1995;15:6987–6998. doi: 10.1523/JNEUROSCI.15-11-06987.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fishell G. Curr Opin Neurobiol. 1997;7:62–69. doi: 10.1016/s0959-4388(97)80121-3. [DOI] [PubMed] [Google Scholar]
- 36.Snyder E Y, Deitcher D L, Walsh C, Arnold-Aldea S, Hartwieg E A, Cepko C L. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 37.Brüstle O, Maskos U, McKay R D G. Neuron. 1995;15:1275–1285. doi: 10.1016/0896-6273(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 38.Raff M C, Barres B A, Burne J F, Coles H S, Ishizaki Y, Jacobson M D. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- 39.Oppenheim R W, Schwartz L M, Shatz C J. J Neurobiol. 1992;23:1111–1115. doi: 10.1002/neu.480230903. [DOI] [PubMed] [Google Scholar]