Abstract
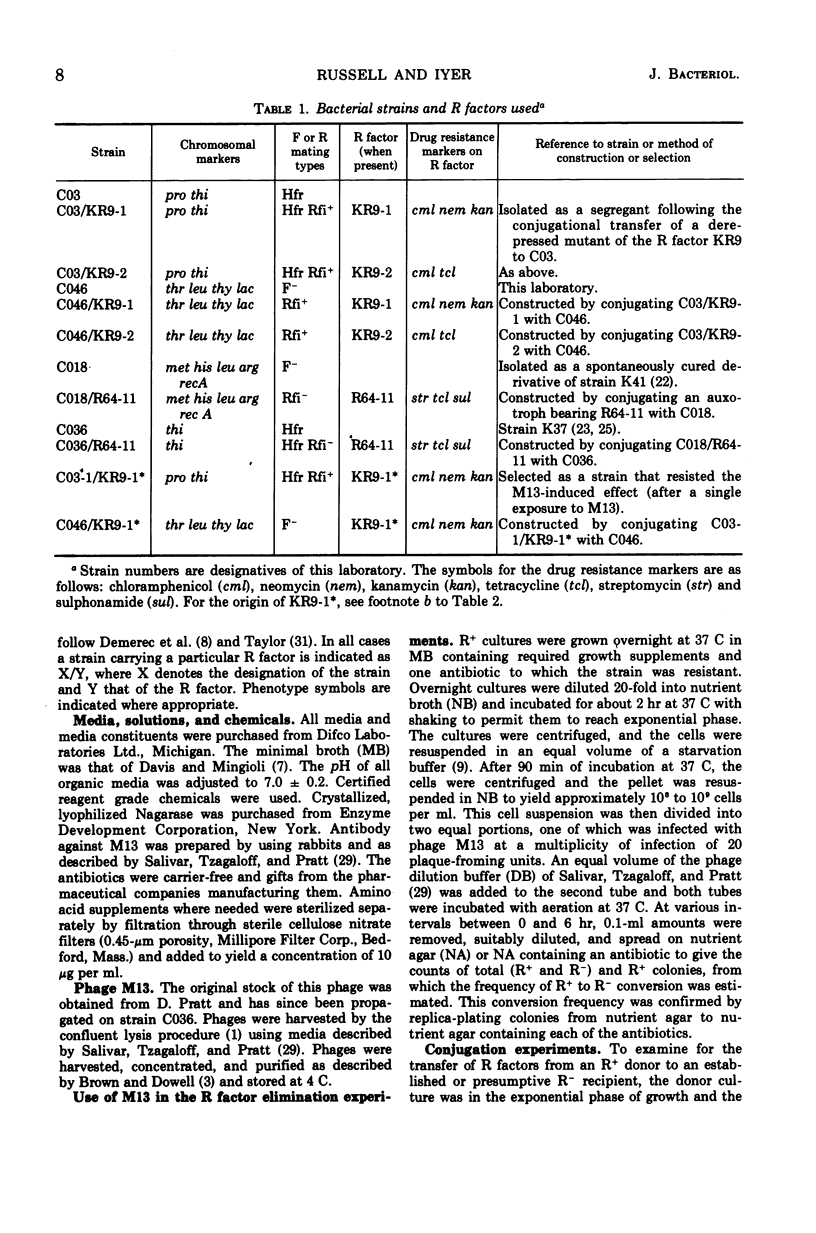
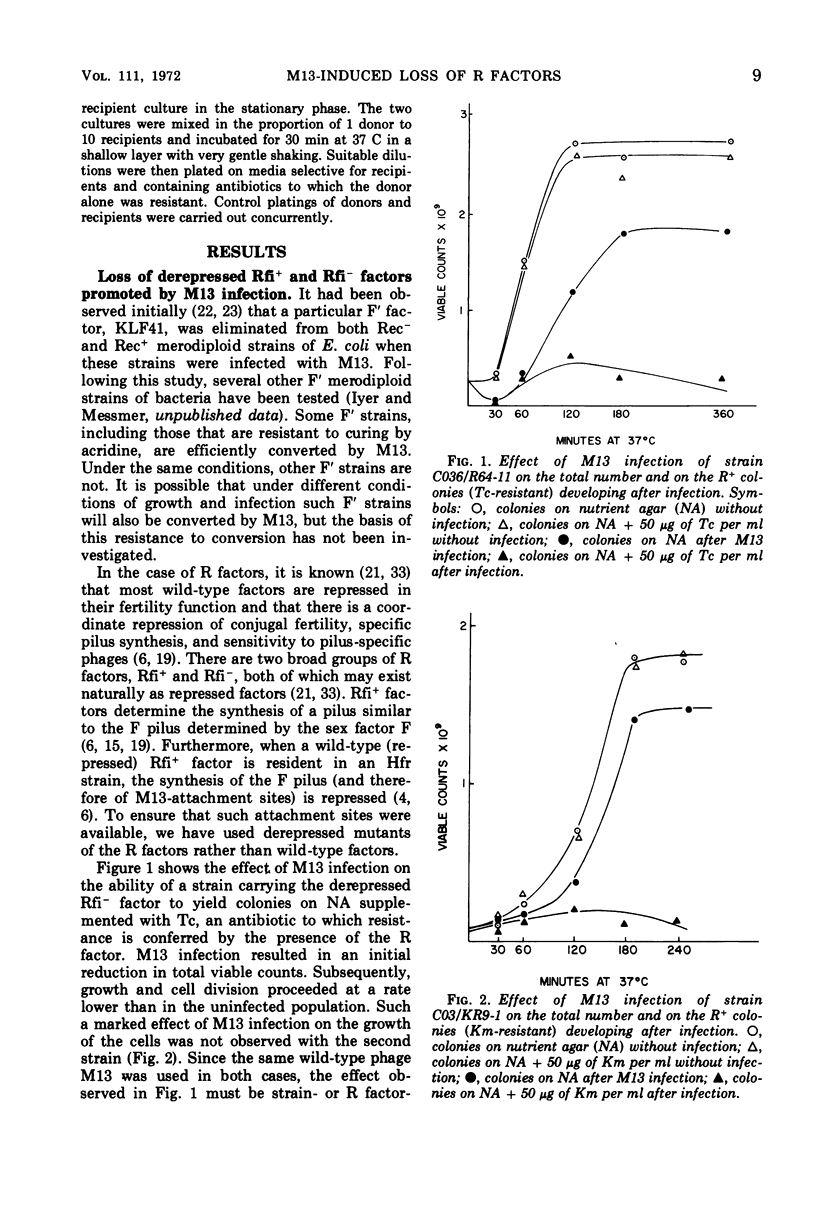
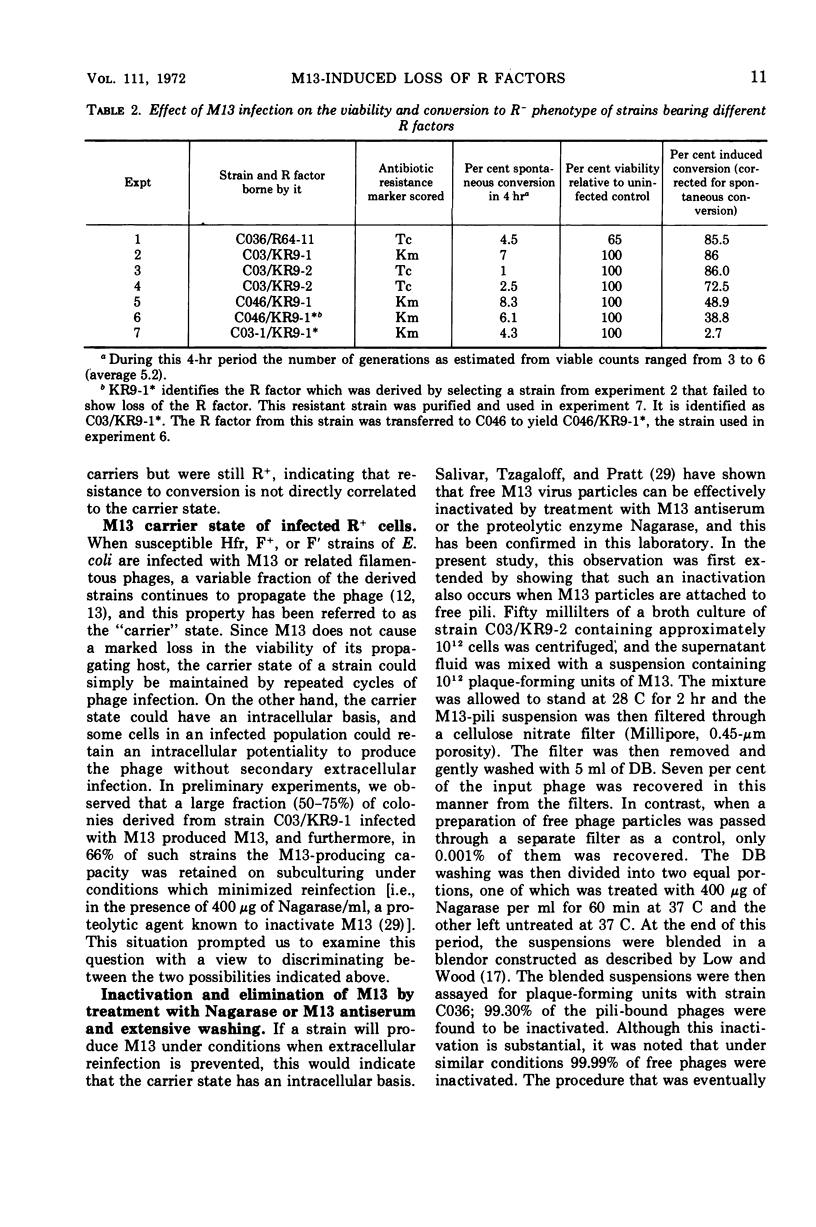
The infection of different Hfr strains of Escherichia coli bearing derepressed R factors of the fi+ or fi− type can result in the loss of the R factor and the conversion of the infected cells to the R− state. This extends earlier observations on the elimination of F′ factors by bacteriophage M13 infection. Variability in the efficiency of this conversion can arise because of genetic factors independent of the R factor being eliminated. A fraction of the infected but unconverted R+ cells were M13 carrier strains. The carrier state had an intracellular basis, and single R+ cells could maintain the carrier state.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E., Dewar C. A. Intracellular changes in cells of Escherichia coli infected with a filamentous bacteriophage. J Gen Virol. 1967 Apr;1(2):179–188. doi: 10.1099/0022-1317-1-2-179. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Dowell C. E. Replication of coliphage M-13. I. Effects on host cells after synchronized infection. J Virol. 1968 Nov;2(11):1290–1295. doi: 10.1128/jvi.2.11.1290-1295.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Thiéry J. On the mode of action of colicins: a model of regulation at the membrane level. J Theor Biol. 1967 Nov;17(2):315–318. doi: 10.1016/0022-5193(67)90175-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Lawn A. M., Meynell E. The relationship of F type piliation and F phage sensitivity to drug resistance transfer in R+F- Escherichia coli K 12. J Gen Microbiol. 1966 Nov;45(2):365–376. doi: 10.1099/00221287-45-2-365. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 3. Phage maturation and lysis after synchronized infection. J Mol Biol. 1965 Jul;12(3):641–646. doi: 10.1016/s0022-2836(65)80318-7. [DOI] [PubMed] [Google Scholar]
- Falaschi A., Kornberg A. A lipopolysaccharide inhibitor of a DNA methyl transferase. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1713–1720. doi: 10.1073/pnas.54.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMANN BERLING H., MAZE R. RELEASE OF MALE-SPECIFIC BACTERIOPHAGES FROM SURVIVING HOST BACTERIA. Virology. 1964 Mar;22:305–313. doi: 10.1016/0042-6822(64)90021-2. [DOI] [PubMed] [Google Scholar]
- HOFFMANN-BERLING H., DUERWALD H., BEULKE I. EIN FAEDIGER DNS-PHAGE (FD) UND EIN SPHAERISCHER RNS-PHAGE (FR) WIRTSSPEZIFISCH FUER MAENNLICHE STAEMME VON E. COLI. III. BIOLOGISCHES VERHALTEN VON FD UND FR. Z Naturforsch B. 1963 Nov;18:893–898. [PubMed] [Google Scholar]
- Hsu Y. C. Propagation or elimination of viral infection in carrier cells. Bacteriol Rev. 1968 Dec;32(4 Pt 1):387–399. [PMC free article] [PubMed] [Google Scholar]
- Iyer R. V., Iyer V. N. Genetic and molecular properties of an infectious antibiotic resistance (R) factor isolated from Klebsiella. J Bacteriol. 1969 Nov;100(2):605–616. doi: 10.1128/jb.100.2.605-616.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOW B., WOOD T. H. A QUICK AND EFFICIENT METHOD FOR INTERRUPTION OF BACTERIAL CONJUGATION. Genet Res. 1965 Jul;6:300–303. doi: 10.1017/s001667230000416x. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- Lawn A. M. Morphological features of the pili associated with Escherichia coli K 12 carrying R factors or the F factor. J Gen Microbiol. 1966 Nov;45(2):377–383. doi: 10.1099/00221287-45-2-377. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Loss of an episomal fertility factor following the multiplication of coliphage M13. Mol Gen Genet. 1969 Oct 13;105(2):131–139. doi: 10.1007/BF00445683. [DOI] [PubMed] [Google Scholar]
- Palchoudhury S. R., Iyer V. N. Non-essentiality of the recA- mutation in the phenomenon of bacteriophage M13-induced elimination of F' factors. J Bacteriol. 1971 Jun;106(3):1040–1042. doi: 10.1128/jb.106.3.1040-1042.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Primrose S. B., Brown L. R., Dowell C. E. Host cell participation in small virus replication. I. Replication of M-13 in a strain of Escherichia coli with a temperature-sensitive lesion in deoxyribonucleic acid synthesis. J Virol. 1968 Nov;2(11):1308–1314. doi: 10.1128/jvi.2.11.1308-1314.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A., Mitra S. Increased fragility of Escherichia coli after infection with bacteriophage M13. J Virol. 1970 Sep;6(3):333–339. doi: 10.1128/jvi.6.3.333-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALIVAR W. O., TZAGOLOFF H., PRATT D. SOME PHYSICAL-CHEMICAL AND BIOLOGICAL PROPERTIES OF THE ROD-SHAPED COLIPHAGE M13. Virology. 1964 Nov;24:359–371. doi: 10.1016/0042-6822(64)90173-4. [DOI] [PubMed] [Google Scholar]
- Schwartz F. M., Zinder N. D. Morphological changes in Escherichia coli infected with the DNA bacteriophage fl. Virology. 1968 Feb;34(2):352–355. doi: 10.1016/0042-6822(68)90246-8. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L. THE VIRAL CARRIER STATE IN ANIMAL CELL CULTURES. Prog Med Virol. 1964;6:111–148. [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman J. Possible allosteric effects in extended biological systems. J Mol Biol. 1969 Feb 14;39(3):523–538. doi: 10.1016/0022-2836(69)90142-9. [DOI] [PubMed] [Google Scholar]


