Abstract
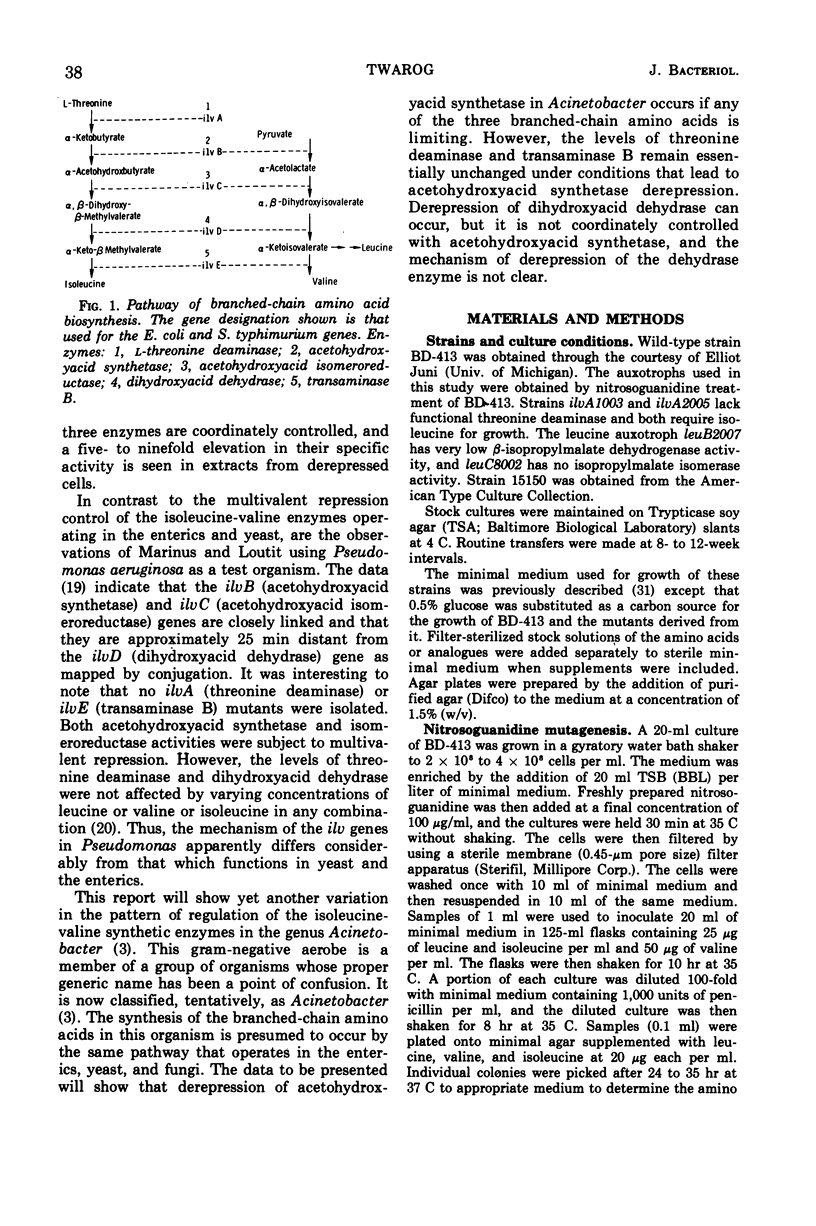
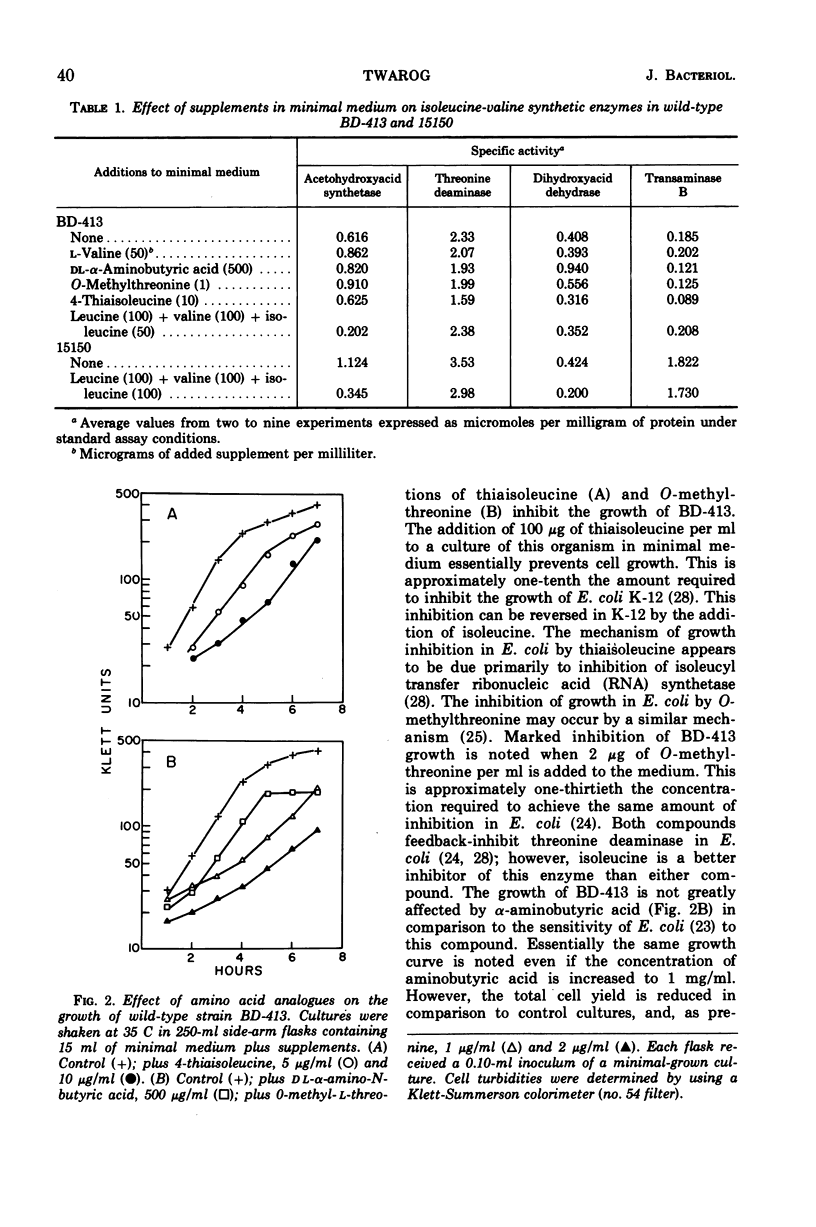
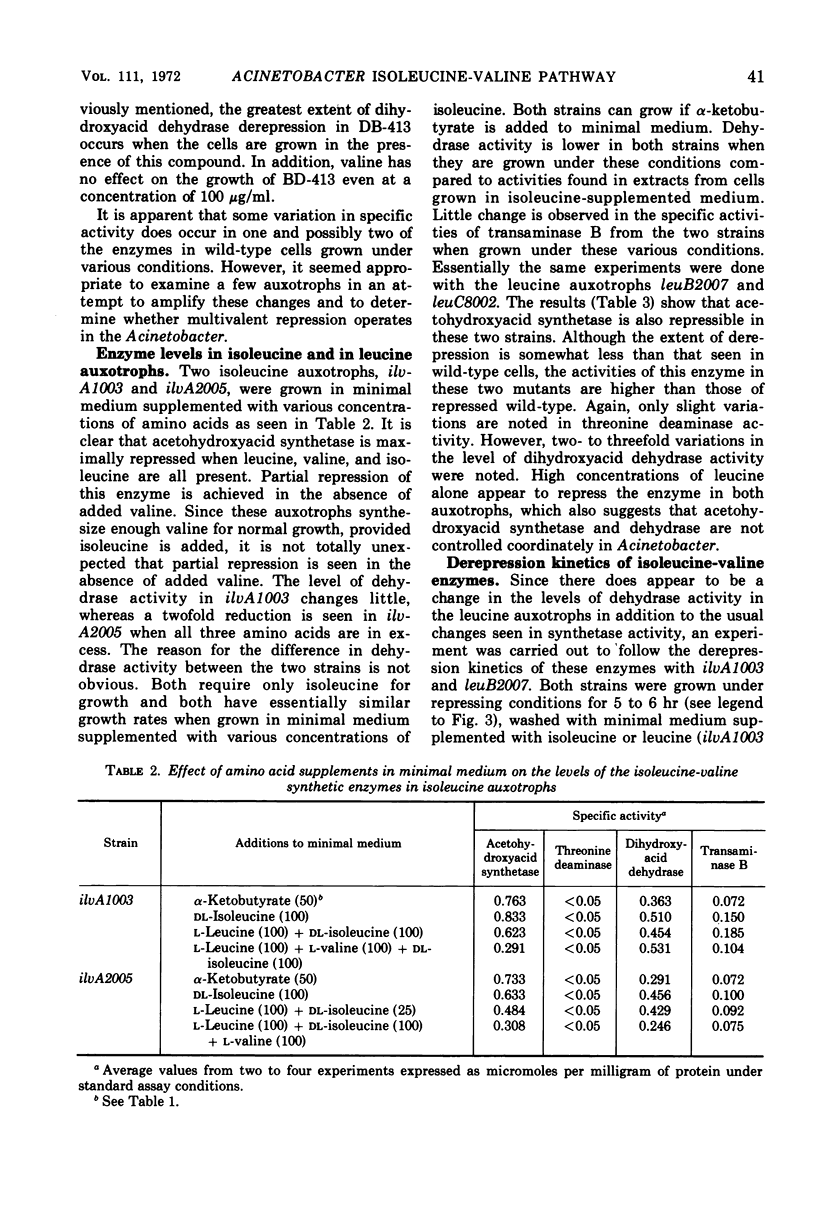
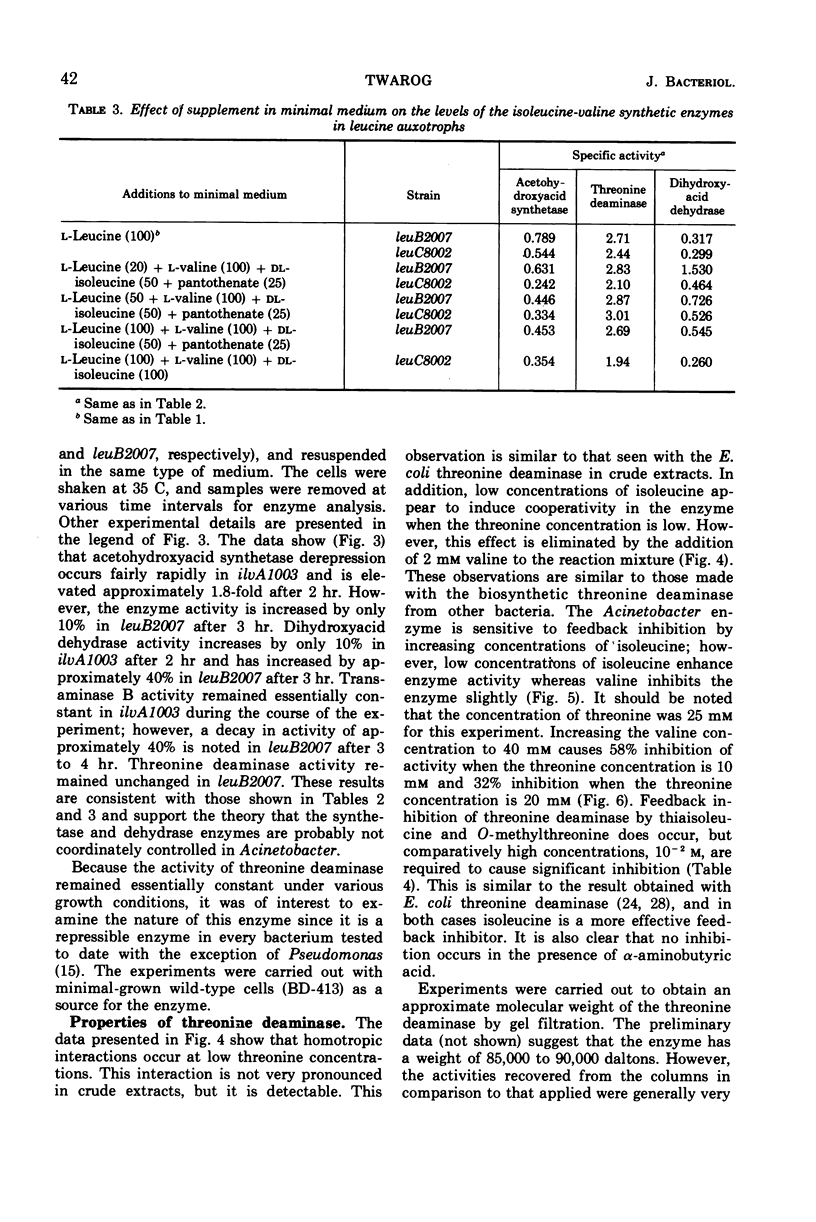
Regulation of four of the enzymes required for isoleucine and valine biosynthesis in Acinetobacter was studied. A three- to fourfold derepression of acetohydroxyacid synthetase was routinely observed in two different wild-type strains when grown in minimal medium relative to cells grown in minimal medium supplemented with leucine, valine, and isoleucine. A similar degree of synthetase derepression was observed in appropriately grown isoleucine or leucine auxotrophs. No significant derepression of threonine deaminase or transaminase B occurred in either wild-type or mutant cells grown under a variety of conditions. Three amino acid analogues were tested with wild-type cells; except for a two- to threefold derepression of dihydroxyacid dehydrase when high concentrations of aminobutyric acid were added to the medium, essentially the same results were obtained. Experiments showed that threonine deaminase is subject to feedback inhibition by isoleucine and that valine reverses this inhibition. Cooperative effects in threonine deaminase were demonstrated with crude extracts. The data indicate that the synthesis of isoleucine and valine in Acinetobacter is regulated by repression control of acetohydroxyacid synthetase and feedback inhibition of threonine deaminase and acetohydroxyacid synthetase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. R., Calvo J. M., Freundlich M. Mutants of Salmonella typhimurium with an altered leucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Apr;106(1):213–220. doi: 10.1128/jb.106.1.213-220.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS R. O., UMBARGER H. E., GROSS S. R. THE BIOSYNTHESIS OF LEUCINE. III. THE CONVERSION OF ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE TO ALPHA-KETOISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1053–1058. doi: 10.1021/bi00905a024. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollon A. P., Magee P. T. Involvement of threonine deaminase in multivalent repression of the isoleucine-valine pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2169–2172. doi: 10.1073/pnas.68.9.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Umbarger H. E. Biosynthesis of branched-chain amino acids in yeast: regulation of synthesis of the enzymes of isoleucine and valine biosynthesis. J Bacteriol. 1969 May;98(2):623–628. doi: 10.1128/jb.98.2.623-628.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S., Armstrong F. B. Branched-chain amino-acid aminotransferase of Salmonella typhimurium. I. Crystallization and preliminary characterization. Biochim Biophys Acta. 1971 Jan 13;227(1):56–66. doi: 10.1016/0005-2744(71)90167-7. [DOI] [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisumi M., Komatsubara S., Chibata I. Multivalent repression and genetic depression of isoleucine-valine biosynthetic enzymes in Serratia marcescens. J Bacteriol. 1971 Sep;107(3):824–827. doi: 10.1128/jb.107.3.824-827.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee P. T., Hereford L. M. Multivalent repression of isoleucine- valine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1969 Jun;98(3):857–862. doi: 10.1128/jb.98.3.857-862.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Loutit J. S. Regulation of isoleucine-valine biosynthesis in Pseudomonas aeruginosa. I. Characterisation and mapping of mutants. Genetics. 1969 Nov;63(3):547–556. doi: 10.1093/genetics/63.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Loutit J. S. Regulation of isoleucine-valine biosynthesis in Pseudomonas aeruginosa. II. Regulation of enzyme activity and synthesis. Genetics. 1969 Nov;63(3):557–567. doi: 10.1093/genetics/63.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. 3. MAP ORDER OF THE STRUCTURAL GENES AND OPERATOR GENES. J Bacteriol. 1965 Mar;89:661–664. doi: 10.1128/jb.89.3.661-664.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulson M. E., Rabinovitz M., Breitman T. R. O-methylthreonine inhibition of growth and of threonine deaminase in Escherichia coli. J Bacteriol. 1967 Dec;94(6):1890–1895. doi: 10.1128/jb.94.6.1890-1895.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber G., Iaccarino M. Biochemical characterization of a mutant isoleucyl-transfer ribonucleic acid synthetase from Escherichia coli K-12. J Bacteriol. 1971 Sep;107(3):828–832. doi: 10.1128/jb.107.3.828-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog R., Liggins G. L. Enzymes of the tryptophan pathway in Acinetobacter calco-aceticus. J Bacteriol. 1970 Oct;104(1):254–263. doi: 10.1128/jb.104.1.254-263.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



