Abstract
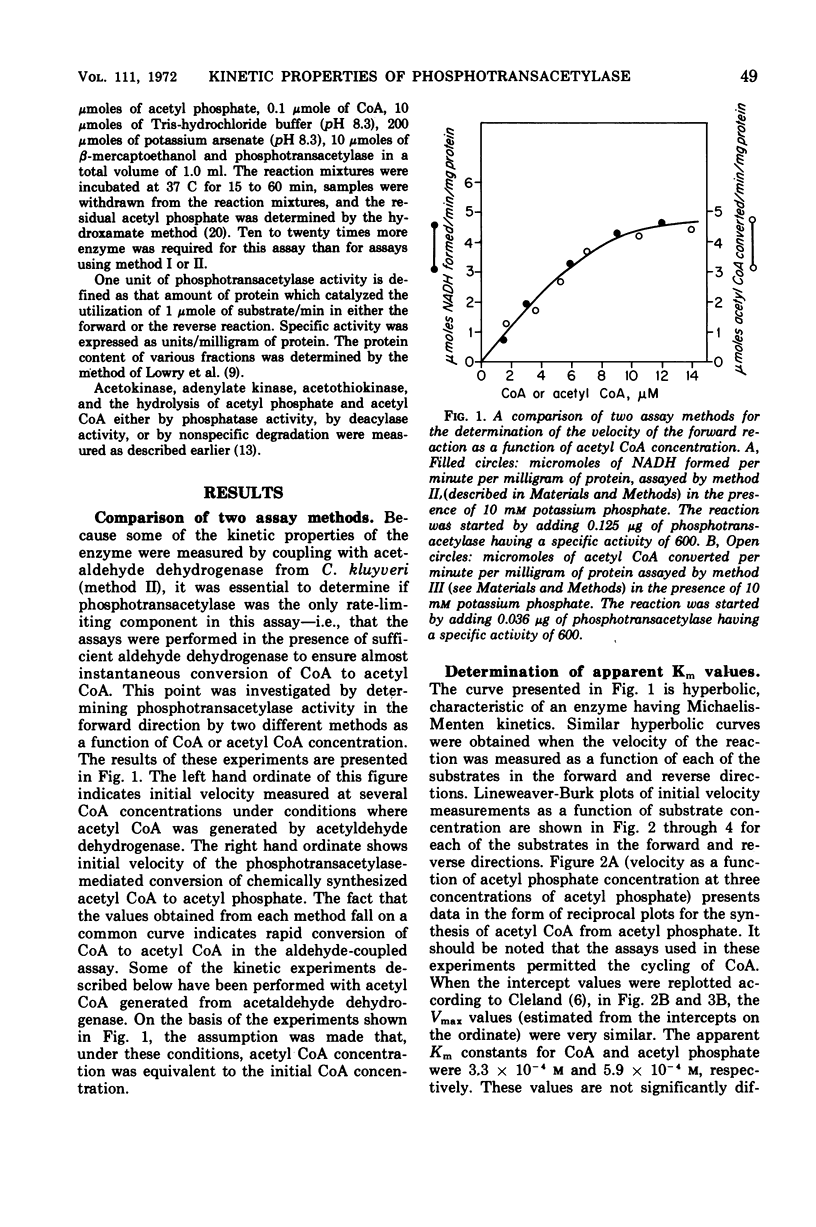
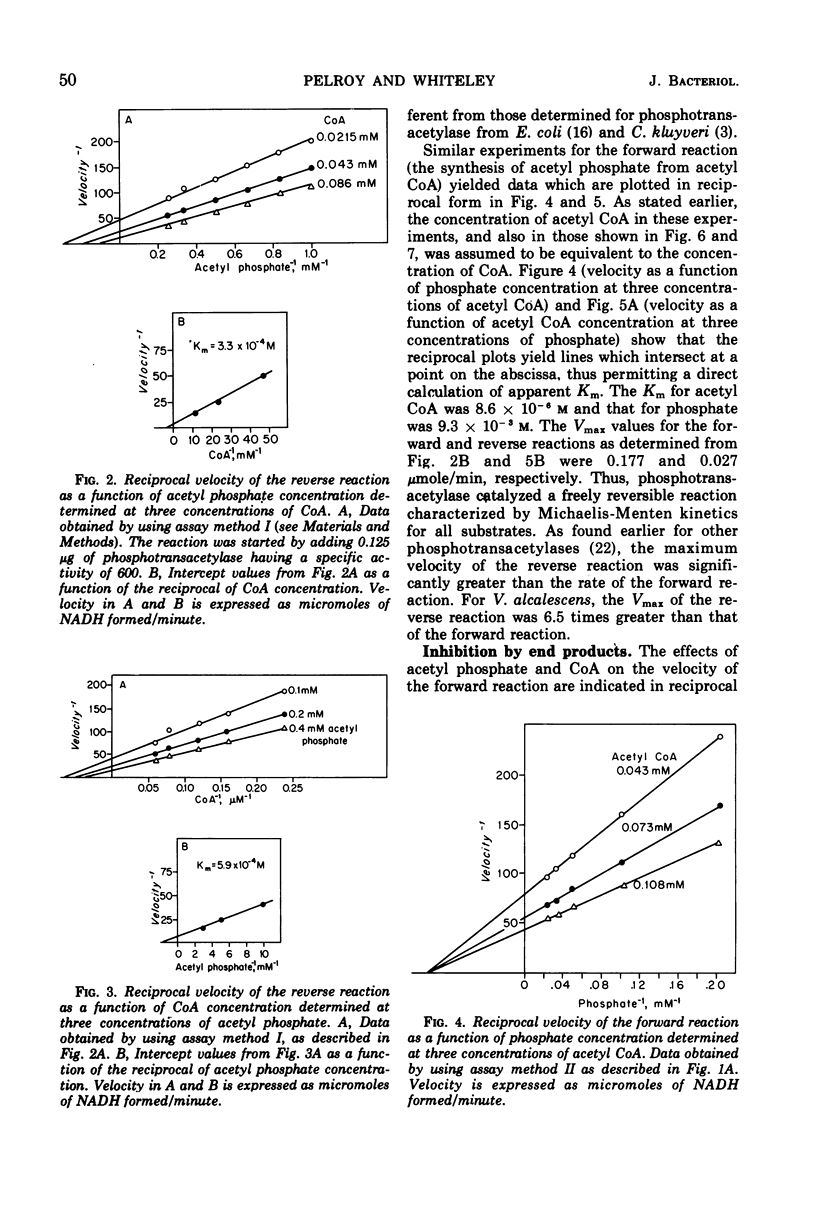
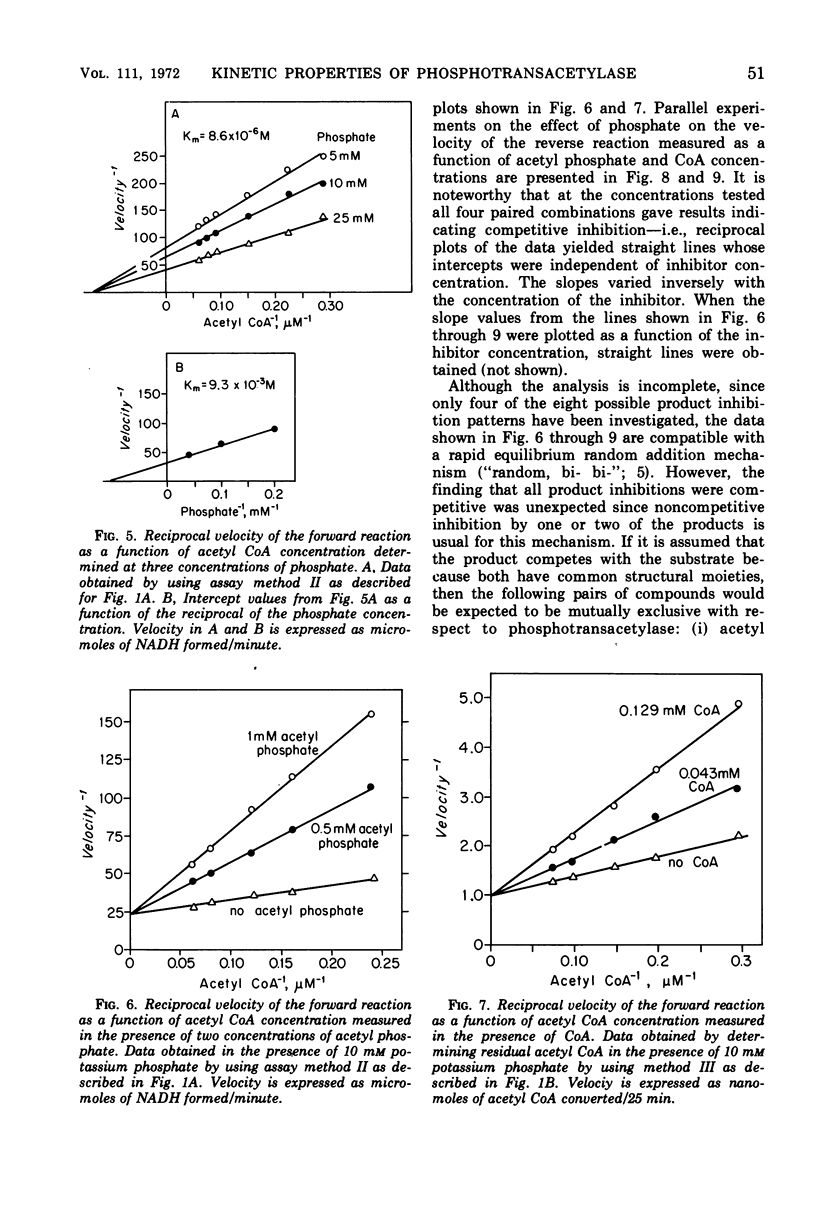
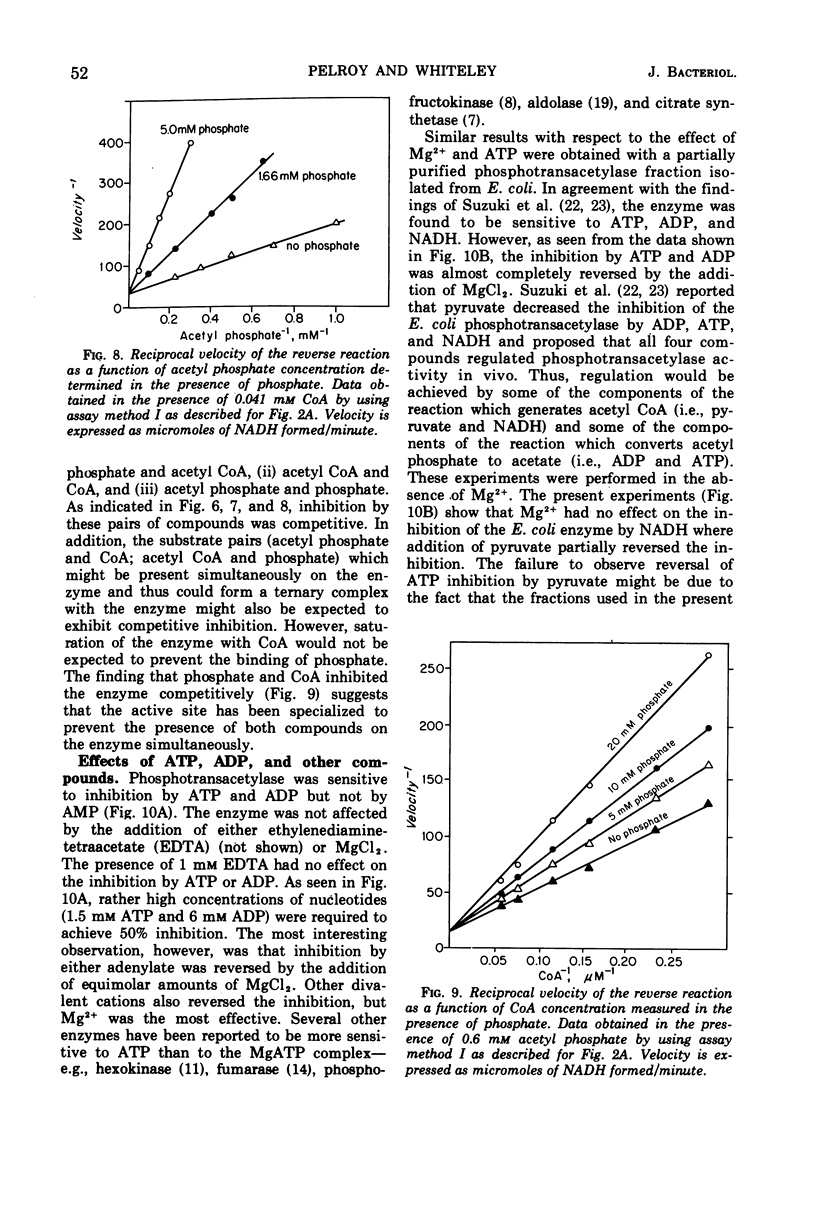
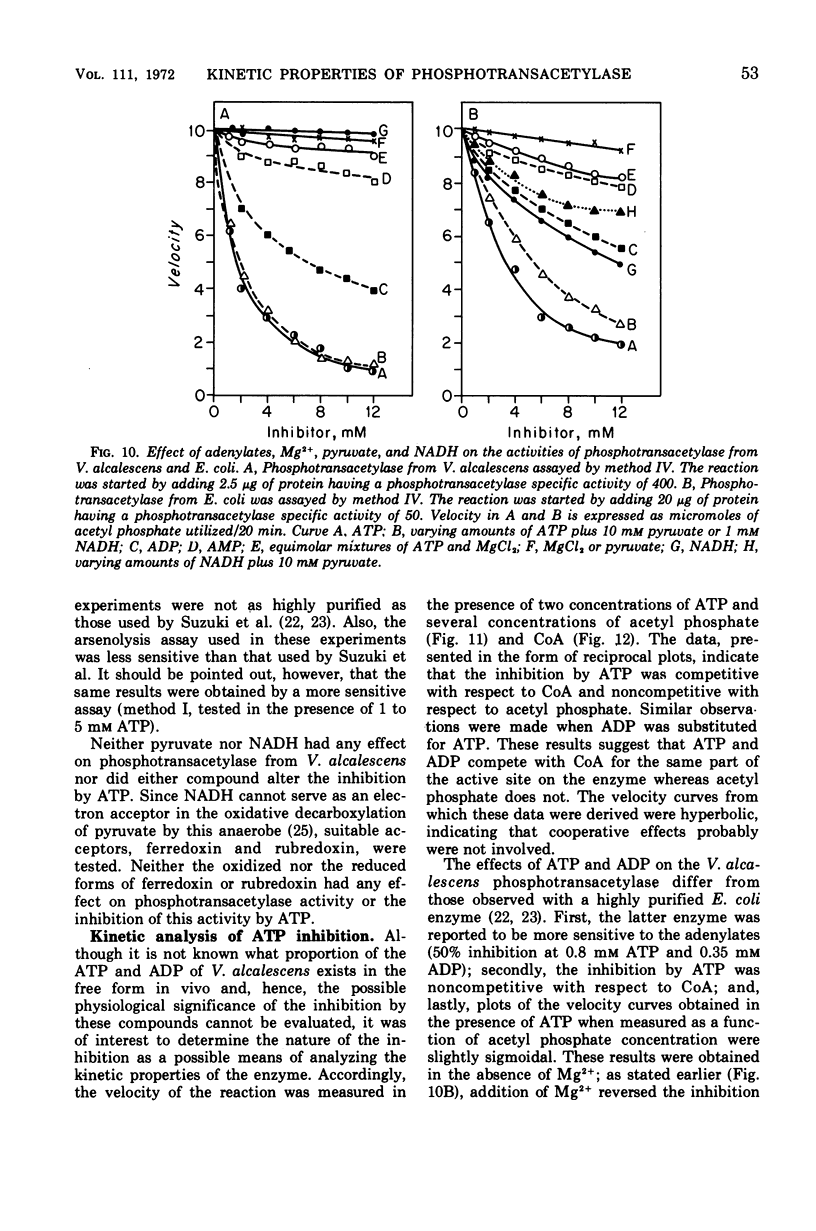
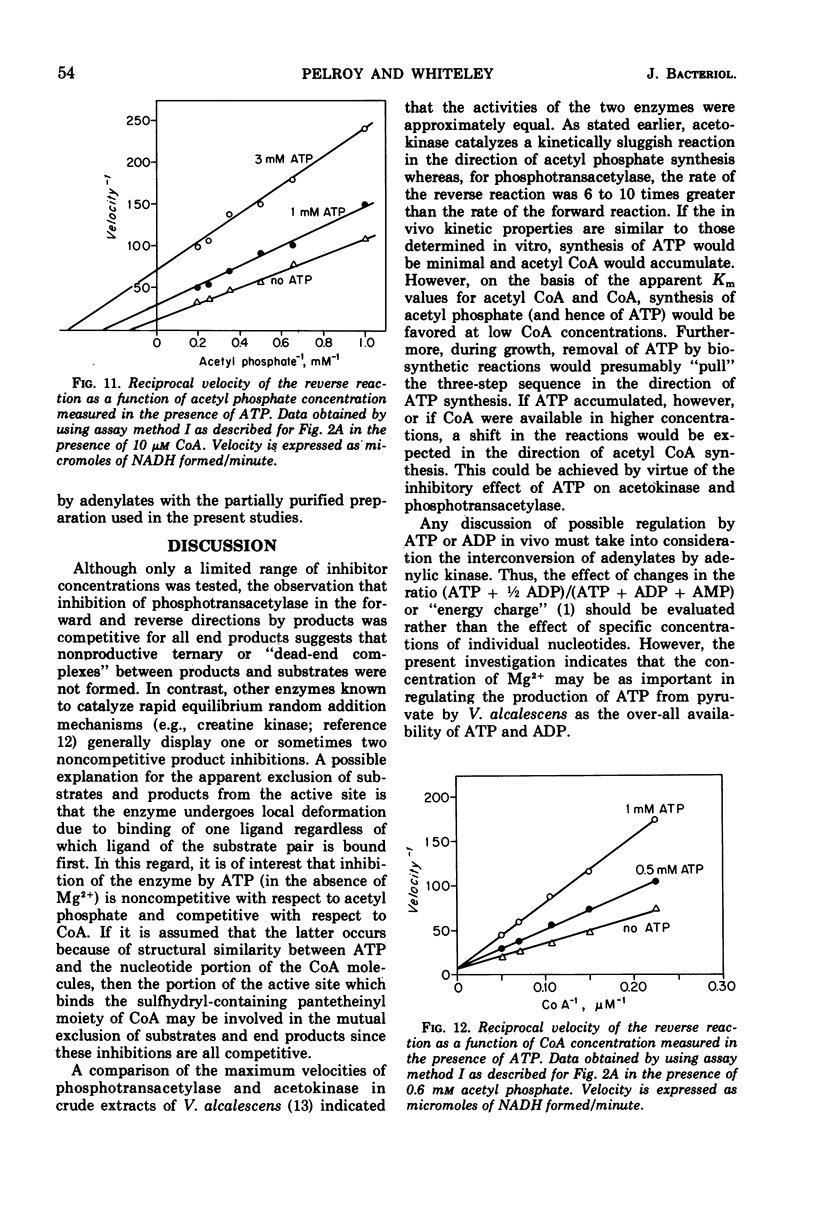
The phosphotransacetylase of Veillonella alcalescens catalyzes a reversible reaction with Michaelis-Menten kinetics for all substrates. The rate of the reverse reaction (the synthesis of acetyl coenzyme A from acetyl phosphate) was 6.5 times greater than the rate of the forward reaction (the synthesis of acetyl phosphate from acetyl coenzyme A). The apparent Km values determined for the forward reaction were 8.6 × 10−6m for acetyl coenzyme A and 9.3 × 10−3m for phosphate. In the reverse reaction, the Km values were 3.3 × 10−4m for coenzyme A and 5.9 × 10−4m for acetyl phosphate. The results of an analysis of the inhibition by end products in the forward and reverse directions were compatible with a random bi- bi- mechanism. The enzyme was inhibited by adenosine triphosphate and adenosine diphosphate but was not affected by reduced nicotinamide adenine dinucleotide or pyruvate. The inhibition by adenosine triphosphate was noncompetitive with respect to acetyl phosphate and competitive with respect to coenzyme A. MgCl2 reversed the inhibition by adenosine triphosphate or adenosine diphosphate. The role of Mg2+ and adenylates in the regulation of phosphotranscetylase activity is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- BERGMEYER H. U., HOLZ G., KLOTZSCH H., LANG G. PHOSPHOTRANSACETYLASE AUS CLOSTRIDIUM KLUYVERI. ZUECHTUNG DES BACTERIUMS, ISOLIERUNG, KRISTALLISATION UND EIGENSCHAFTEN DES ENZYMS. Biochem Z. 1963;338:114–121. [PubMed] [Google Scholar]
- Bachmayer H., Piette L. H., Yasunobu K. T., Whiteley H. R. The binding sites of iron in rubredoxin from Micrococcus aerogenes. Proc Natl Acad Sci U S A. 1967 Jan;57(1):122–127. doi: 10.1073/pnas.57.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair J. M. Magnesium, potassium, and the adenylate kinase equilibrium. Magnesium as a feedback signal from the adenine nucleotide pool. Eur J Biochem. 1970 Apr;13(2):384–390. doi: 10.1111/j.1432-1033.1970.tb00940.x. [DOI] [PubMed] [Google Scholar]
- KOIKE M., REED L. J., CARROLL W. R. alpha-Keto acid dehydrogenation complexes. IV. Resolution and reconstitution of the Escherichia coli pyruvate dehydrogenation complex. J Biol Chem. 1963 Jan;238:30–39. [PubMed] [Google Scholar]
- Kosicki G. W., Lee L. P. Effect of divalent metal ions on nucleotide inhibition of pig heart citrate synthase. J Biol Chem. 1966 Aug 10;241(15):3571–3574. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Passonneau J. V. Kinetic evidence for multiple binding sites on phosphofructokinase. J Biol Chem. 1966 May 25;241(10):2268–2279. [PubMed] [Google Scholar]
- Lusk J. E., Williams R. J., Kennedy E. P. Magnesium and the growth of Escherichia coli. J Biol Chem. 1968 May 25;243(10):2618–2624. [PubMed] [Google Scholar]
- MELCHIOR N. C., MELCHIOR J. B. The role of complex metal ions in the yeast hexokinase reaction. J Biol Chem. 1958 Apr;231(2):609–623. [PubMed] [Google Scholar]
- Morrison J. F., James E. The mechanism of the reaction catalysed by adenosine triphosphate-creatine phosphotransferase. Biochem J. 1965 Oct;97(1):37–52. doi: 10.1042/bj0970037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelroy R. A., Whiteley H. R. Regulatory properties of acetokinase from Veillonella alcalescens. J Bacteriol. 1971 Jan;105(1):259–267. doi: 10.1128/jb.105.1.259-267.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner P. E., Cohen L. H. Effects of adenosine triphosphate and magnesium ions on the fumarase reaction. J Biol Chem. 1969 Feb 10;244(3):1070–1075. [PubMed] [Google Scholar]
- SPOLTER P. D., ADELMAN R. C., WEINHOUSE S. DISTINCTIVE PROPERTIES OF NATIVE AND CARBOXYPEPTIDASE-TREATED ALDOLASES OF RABBIT MUSCLE AND LIVER. J Biol Chem. 1965 Mar;240:1327–1337. [PubMed] [Google Scholar]
- Schwartz E. R., Reed L. J. Regulation of the activity of the pyruvate dehydrogenase complex of Escherichia coli. Biochemistry. 1970 Mar 17;9(6):1434–1439. doi: 10.1021/bi00808a019. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Suzuki T., Kameda K. Y., Abiko Y. Phosphotransacetylase of Escherichia coli B, purification and properties. Biochim Biophys Acta. 1969;191(3):550–558. doi: 10.1016/0005-2744(69)90348-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Abiko Y., Shimizu M. Activation and inhibition of purified phosphotransacetylase of Escherichia coli B by pyruvate and by NADH2 and certain nucleotides. Biochem Biophys Res Commun. 1969 Apr 10;35(1):102–108. doi: 10.1016/0006-291x(69)90488-4. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Phosphotransacetylase of Escherichia coli B, activation by pyruvate and inhibition by NADH and certain nucleotides. Biochim Biophys Acta. 1969;191(3):559–569. doi: 10.1016/0005-2744(69)90349-0. [DOI] [PubMed] [Google Scholar]
- Tsunoda J. N., Yasunobu K. T., Whiteley H. R. Non-heme iron proteins. IX. The amino acid sequence of ferredoxin from Micrococcus aerogenes. J Biol Chem. 1968 Dec 10;243(23):6262–6272. [PubMed] [Google Scholar]
- WHITELEY H. R., McCORMICK N. G. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J Bacteriol. 1963 Feb;85:382–393. doi: 10.1128/jb.85.2.382-393.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R., Pelroy R. A. Purification and properties of phosphotransacetylase from Veillonella alcalescens. J Biol Chem. 1972 Mar 25;247(6):1911–1917. [PubMed] [Google Scholar]


