Abstract
Hearing is one of the last sensory modalities to be subjected to genetic analysis in Drosophila melanogaster. We describe a behavioral assay for auditory function involving courtship among groups of males triggered by the pulse component of the courtship song. In a mutagenesis screen for mutations that disrupt the auditory response, we have recovered 15 mutations that either reduce or abolish this response. Mutant audiograms indicate that seven mutants reduced the amplitude of the response at all intensities. Another seven abolished the response altogether. The other mutant, 5L3, responded only at high sound intensities, indicating that the threshold was shifted in this mutant. Six mutants were characterized in greater detail. 5L3 had a general courtship defect; courtship of females by 5L3 males also was affected strongly. 5P1 males courted females normally but had reduced success at copulation. 5P1 and 5N18 showed a significant decrement in olfactory response, indicating that the defects in these mutations are not specific to the auditory pathway. Two other mutants, 5M8 and 5N30, produced amotile sperm although in 5N30 this phenotype was genetically separable from the auditory phenotype. Finally, a new adult circling behavior phenotype, the pirouette phenotype, associated with massive neurodegeneration in the brain, was discovered in two mutants, 5G10 and 5N18. This study provides the basis for a genetic and molecular dissection of auditory mechanosensation and auditory behavior.
Hearing is one of the mechanosensory modalities. In contrast to other senses, such as sight, smell, and taste, in which the sensory transduction pathways have been characterized at the molecular level, relatively little is known about molecular transduction machinery in the mechanical senses. Recently, a bacterial mechanosensory channel molecule was cloned, and progress has been made in the touch system of Caenorhabditis elegans, in which genetic and molecular analyses have led to the characterization of several transduction proteins (reviewed in ref. 1). In Drosophila, screens for mutations that affect touch sensitivity have been undertaken (2) although none of the corresponding genes has yet been cloned. Several murine and human genes that cause syndromic or nonsyndromic deafness have now been identified (reviewed in refs. 3 and 4), but many remain uncharacterized.
Multicellular organisms detect airborne sound with a transformer structure, usually either a tympanal membrane or a flagellum, that resonates in the presence of the sound, transforming the sound energy into a mechanical signal. This mechanical signal is transferred to one or more sensory cells, often through mechanical linkages that may amplify the signal. The sensory cells transduce the mechanical signal into an electrochemical signal within the cell. For the sensory information regarding the airborne sounds in the environment to be useful to the organism, the information from the sensory cells and other relevant information is assimilated in the central nervous system, where an appropriate response is initiated.
Our understanding of the Drosophila auditory system comes from several decades of work on courtship. Wing-generated auditory cues are involved in courtship, along with visual, olfactory, gustatory, and tactile signals (see ref. 5 for review). Three components of auditory communication have been identified. The main component, the pulse song (6–8) that the male produces with his outstretched wing, consists of bursts of pulses with a mean interpulse interval of ≈34 ms (Fig. 1C) that stimulates both partners in the courtship, thereby reducing the latency to copulation. Females respond best to songs in which the interpulse interval oscillates rhythmically (refs. 9 and 10; cf. refs. 11 and 12). The courtship song contains a second component, the sine song (13), a 160-Hz sinusoidal hum. The sine song appears to function as a precourtship stimulatory signal (14). A third sound, the rejection signal, is correlated with the behavior of unreceptive flies as they swat the courting fly away with their wings (15) although it has not been tested whether the sound alone will inhibit a courting male.
Figure 1.
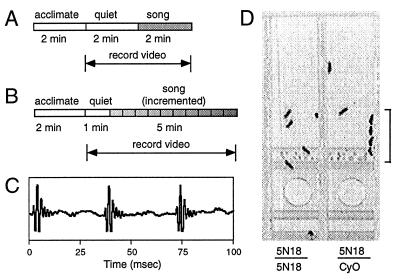
Auditory behavior assay. The pulse song triggers groups of males to court one another (13). Groups of six males were tested for this auditory response in Plexiglas chambers with nylon mesh at the ends to allow acoustic stimulation from the speaker, and the behavior was videotaped. (A and B) Acoustic stimulation protocols for the single intensity test (A) and for the audiogram, or incremented intensity, test (B). (C) Trace of computer-synthesized pulse song as recorded from the speaker at playback. (D) A video frame of 5N18 mutant homozygotes (left) and heterozygous controls (right) during presentation of song. The chain of courting males (bracket) was scored as 4 in the audiogram.
Although several mutations have been identified that affect the generation of the song (reviewed in ref. 5), our understanding of the reception of the song would be greatly advanced by characterizing mutations that disrupt responses to airborne sound. Here we describe an auditory behavior assay and its use in a mutagenesis screen to identify such mutants. Because auditory behavior is a functional test, no assumptions regarding the identity of the auditory organ are made in a mutagenesis screen. However, the main receptor for courtship songs is thought to be in the antenna (16–20) with the arista acting as the transformer structure (17). The auditory mechanoreceptors are most likely the chordotonal sensilla of Johnston’s organ in the second antennal segment (16, 21), which are arranged to detect articulation of the third antennal segment relative to the second.
The nature of the molecular machinery responsible for mechanotransduction in Johnston’s organ, the information coding mechanisms and the neuronal circuitry responsible for transmitting the sensory signals to central and motor parts of the auditory behavior pathways, are largely uncharacterized. To approach these mechanisms at the molecular level, we have undertaken a genetic analysis of the Drosophila auditory system. The classical assay for auditory function in Drosophila is female receptivity, measured as a statistical difference in latency to copulation, and would be difficult to use in a genetic screen. Instead, we based our assay on the observation of von Schilcher (13), who found that a group of males will vigorously court one another if presented with the pulse song. Several types of mutations might be expected, including dominant mutations that disrupt the behavior in heterozygous condition, recessive mutations that disrupt components entirely specific to the auditory machinery, or recessive mutations that affect other structures or processes in addition to the auditory system. We expect that mutations in genes specifically involved in the auditory response will be viable because atonal (ato) mutant flies, in which all chordotonal organs including Johnston’s organ fail to develop, are viable although rather uncoordinated (22). In addition, we expect to recover hypomorphic mutations that disrupt primarily the auditory function of pleiotropic genes and those dominant mutations that are not homozygous lethal. This study provides the basis for a systematic approach to auditory genetics in Drosophila.
MATERIALS AND METHODS
Genetic Strains and Mutagenesis.
The background strain used for most experiments was w; P{neo, FRT}40A P{w+, FRT}G13, abbreviated 40A-G13. The second chromosome in this strain carries, at the base of each arm, a P transposon containing a yeast FLP recombinase target site (23). In many experiments, the Canton-S wild-type strain also was tested. To identify mutants that affect auditory behavior, males homozygous for the 40A-G13 chromosome were mutagenized with ethyl methane sulfonate (24), and individual second chromosome lines were established by crossing several mutagenized males to w; Sco/CyO females in bottles, back-crossing CyO-bearing offspring in individual vials to the balancer stock and then inbreeding the resulting CyO-bearing offspring. Lines in which the mutagenized 40A-G13 chromosome survived in homozygous condition were selected for analysis. The frequency of lethals in this mutagenesis was ≈60%. Second chromosomes recovered from a particular bottle of mutagenized males were given the same letter designation to indicate possible clustering. Genetic mapping was relative to the dominantly marked Sp Bl Lrm Bc Pu2 chromosome or the recessively marked al dp b pr c px sp chromosome.
Behavior Apparatus.
A Plexiglas behavior chamber with eight parallel cells was constructed with inside dimensions 5 × 1 × 0.6 cm. Both end walls of each cell were made of fine nylon mesh to allow horizontal presentation of sound from an external speaker (Radio Shack 8" 4-Ω speaker) whose cone was 15 cm from the center of the chamber. Sound was generated with labview 2 virtual instrument software (National Instruments, Austin, TX) on a Macintosh Quadra 800 computer fitted with a LabNB AD/DA converter board (National Instruments), recorded onto a standard audio cassette tape, then replayed to the speaker through a Realistic MPA-30 amplifier (Radio Shack, Ft. Worth, TX). A Nikon VN-720 Hi8 format video camera was mounted above the chamber to record the behavior.
Pulses were generated by multiplying 2.5 cycles of a sine wave with an envelope of one cycle (trough-to-trough) of a sine wave of the same total length (5 ms). Pulse song consisted of these pulses interspersed with quiet intervals of 30 ms. Intermittent pulse song consisted of 2 s of song alternating with 3 s of silence. Sine song was at a frequency of 160 Hz, and white noise was uniform white noise.
Auditory Behavior Assay.
Males were collected under ether anesthesia, and wings and, when necessary, aristae were removed at this time with sharpened forceps. All flies, whether surgically treated or not, were housed in vials in the groups with which they were to be tested 3–7 days later. This time allows wounds to heal and deprives the males of courtship stimuli from females. Flies were transferred to fresh vials every 2–3 days but not on the day of testing.
To perform the assay, six wingless males of a given genotype were introduced with a mouth pipette (to avoid anesthesia) into each cell of the behavior chamber and given 2 min to settle down (Fig. 1A) before video recording.
For initial mutagenesis screening, a single sound intensity of 80 dB was used (Fig. 1A), and the response was scored subjectively by using a single run of six flies per line. Those lines that gave a clear robust response were immediately discarded whereas the remaining lines were retested at least once. For further characterization, audiograms were measured by quantitation of the behavioral response using the incremented auditory stimulation paradigm (Figs. 1B and 2A). Courtship was quantified by scoring the number of males involved in courtship during each 3-s interval and summing these scores for each 30-s increment for a maximum courtship index of 60. Courtship events scored were “following” and “orientation,” and, for ease of scoring, passive recipients were included in the score; for example, the right cell in Fig. 1D was scored as 4.
Figure 2.
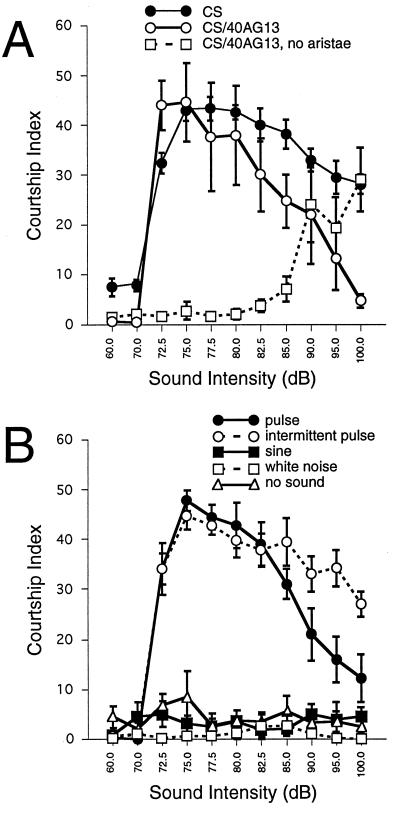
Wild-type audiograms. (A) Computer-generated continuous pulse song was played back at 30-s increments of sound intensity. The background noise level in the room was ≈60 dB. Sound intensity doubles approximately every 3 dB. Courtship was quantified with a maximum courtship index of 60 if all males were courting in every 3-s interval. Males were from the Canton-S wild-type strain or hybrids between Canton-S and the 40A-G13 strain used for mutagenesis. Means ± SEM for three runs are plotted. (B) Male courtship is triggered only by the pulse song, whether presented as a continuous train of pulses or as 2-s bursts of pulse song alternating with 3 s of silence. Neither sine song nor uniform white noise elicited courtship. Means ± SEM for four runs are plotted.
Olfactory Trap Assay.
Tests of olfactory behavior were carried out as described (25). In brief, the trap was made of a 1.5-ml microfuge tube containing a plug of standard Drosophila medium as the olfactory attractant. The bottom of the tube was removed, and a trimmed yellow micropipette tip was inserted into it. The large end of a second trimmed yellow tip was fitted over the first tip to make a long tunnel for the flies to navigate on the basis of smell. This trap was placed in a 100-mm Petri dish containing 10 ml of 1% agarose, and 10 flies were introduced into each dish after light CO2 anesthetization. The number of flies in each trap after 60 h was plotted. Data were analyzed statistically using the t test.
Copulation and Fertility Tests.
Homozygous mutant males were tested for courtship of 3- to 5-day-old virgin Canton-S females by introducing five males and eight females into each cell of the auditory behavior chamber, videotaping for 30 min, and scoring the number of males that copulated with females. After the test, the flies from a given cell were transferred to a vial and scored for the production of larvae over the next week. When males proved sterile, young males were sequestered from females for 24 h, then dissected in PBS to examine testis morphology and sperm motility.
RESULTS
Mutagenesis Screen.
Ethyl methanesulfonate-mutagenized, homozygous viable second chromosome lines were generated for the auditory behavior screen. The frequency of lethal chromosomes was ≈60%. For rapid screening, we used the stimulation protocol in Fig. 1A, which uses a single intensity (80 dB) of computer-generated pulse song (Fig. 1C). This is approximately the sound intensity to which a female would be subjected by a courting male although it may be as high as 95 dB from a male circling her head (26). A video frame of two cells (Fig. 1D) shows several control males courting in a chain in response to the pulse song. Each mutant line was given a subjective qualitative score. Of nearly 400 homozygous viable lines tested, ≈90% gave a robust response in the first round of testing (Table 1) and were discarded. After some retesting, 15 lines displayed consistent reduction or failure in the response and were retained for further characterization.
Table 1.
Mutagenesis screen results
| Total homozygous viable lines screened | 378 | |
| Putative mutant lines retained in first round | 42 | |
| Confirmed mutants | 15 | |
| Reduced response | 7 | |
| No response | 8 |
Audiograms.
To begin categorizing the mutations regarding the possible nature of their defects, we used the incremented sound intensity protocol (Fig. 1B) to quantify the response of each mutant, and we called this detailed response the “audiogram” (Fig. 2). In the wild-type audiogram, there was a threshold at ≈72 dB. At high sound intensities, the response was reduced, consistent with the observations of von Schilcher (13). We have determined that the decreased response was due to decreased sensitivity rather than to fatigue or adaptation by testing groups of flies with individual intensities but with the same time course as the incremented assay. Flies tested at low or intermediate intensities maintained a high level of courtship throughout the experiment whereas those groups tested at the highest intensities responded only modestly throughout the experiment (data not shown).
This response appears to be mediated by the antenna because removal of the aristae abolished the auditory response at the low and intermediate sound intensities although at the high intensities these flies did begin to respond (Fig. 2A). Furthermore, the behavioral response was specific to the pulse song. The response of wild-type flies was very robust when auditory stimulation was done with the pulse song whether it was presented as a continuous train of pulses or intermittent bursts (Fig. 2B). However, the sine song and uniform white noise were completely ineffectual in eliciting a response in this assay.
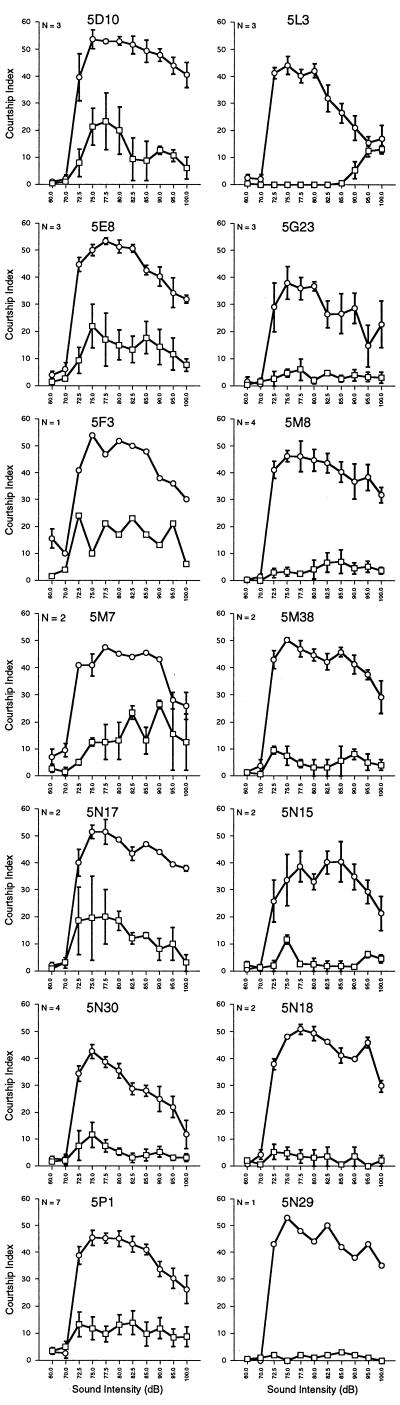
We determined the audiograms for each line (Fig. 3) retained in the primary screening. Several of the mutations showed essentially no response (5G10, 5G23, 5M8, 5M38, 5N15, 5N18, and 5N29) at any of the sound intensities. This indicated that the general auditory pathway was blocked and did not point to any particular step. Most of the remaining mutations caused a reduced response with no intensity-specific effects (5D10, 5E8, 5F3, 5M7, 5N17, 5N30, and 5P1). However, one mutation, 5L3, did not respond at the low and intermediate intensities but did respond at the high intensities. This closely resembled the pattern observed in flies with their aristae removed (Fig. 2A). The external morphology of the aristae in 5L3 mutant flies appeared normal. The high intensity response was not a delayed response to the lower intensities because 5L3 flies presented immediately with high intensity pulse song responded immediately (data not shown).
Figure 3.
Audiograms of 14 mutants. For each mutation, audiograms are shown for the mutant homozygotes (squares) and their balancer heterozygote controls (circles). Means ± SEM for N runs are plotted. For other details, see legend of Fig. 2. The audiogram for pir (5G10) was not determined.
Other Tests.
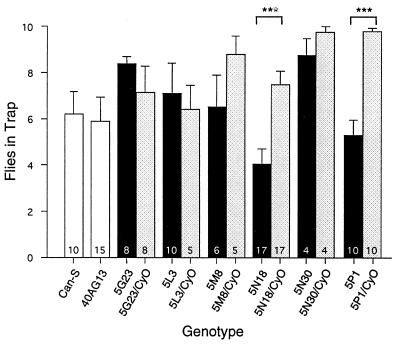
To determine the degree of specificity of these mutants for the auditory system, we tested for effects on a behavior based on a different sensory modality. We chose to test olfactory acuity using the olfactory trap test (25). Fig. 4 shows the results of this test on six of the mutations recovered in the auditory screen. Four of these mutations showed an olfactory response not significantly different from their corresponding heterozygous controls, indicating that these mutations leave the olfactory system largely intact. The two exceptions, 5N18 and 5P1, most likely represent more general defects. Consistent with this, 5N18 flies several weeks old sometimes showed circling behavior that was associated with morphological defects in the brain (see Circling Behavior Mutations; Fig. 6 G and H).
Figure 4.
Olfactory behavior test. Olfactory traps were constructed as described (25) by using standard Drosophila medium as the attractant. Ten males of the indicated genotype were placed with each trap, and the numbers that entered the trap in 60 h were plotted. White bars, wild-type strains; black bars, homozygous mutants; shaded bars, balancer heterozygous controls. The number at the base of each bar indicates the number of traps tested. There were no significant differences except that the olfactory responses of 5N18 and 5P1 homozygotes were reduced significantly (∗∗∗, P < 0.001).
Figure 6.
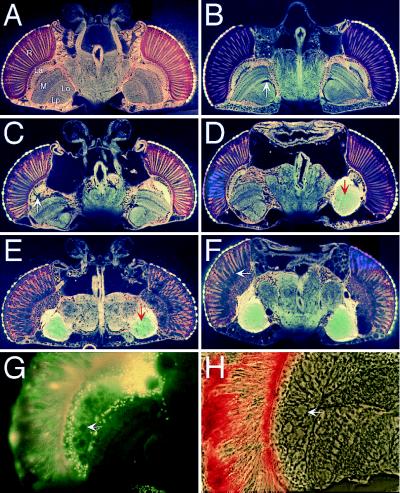
Brain morphology of circling mutants. Horizontal plastic-embedded (Spurr’s medium) heads were sectioned at 5–8 μm and photographed by using autofluorescence. (A) Wild-type head. Optic lobes: R, retina; La, lamina; M, medulla; Lo, lobula; Lp, lobula plate. (B) pir homozygote (18 days old) with no behavioral defect and very slight morphological defect (arrow). (C and D) pir homozygotes (1 day old) that showed the about-face behavior shown in Fig. 5B. Degeneration of the brain was often asymmetrical. Defects were particularly apparent in the optic lobes, such as the lamina (arrow in C). In D, the optic lobes have coalesced into a common sack of amorphous tissue (arrow). (E and F) pir homozygotes (4 days old) that exhibited the severe spinning behavior shown in Fig. 5D. Degeneration was much more severe, again with coalesced optic lobes (arrow in E), and cohesion between subregions of the brain was reduced. All neural connections from the retina to the optic lobes of the brain have been lost, the basement membrane has dissociated from the retina, and photoreceptors have dissociated from one another (arrow in F). (A, C, and E) Sections at the level of the antennal nerve. (D and F) More dorsal sections. (G and H) Section of a 6-week-old 5N18 homozygote that walked in small circles; G shows autofluorescence view, and H shows phase–contrast view. Vacuole-like holes are seen in the lamina (arrows) and other optic lobes, and the integrity of the retina is reduced.
Although the pulse song triggers groups of males to court one another, the ability to court females should not be strongly impaired if the male is unable to hear. Table 2 shows that four of the six mutants tested showed strong courtship of females. The 5L3 mutant, which showed intensity-dependent response in the audiogram, did not copulate with females during the test although, if left with the females, some of the flies would eventually copulate and produce offspring. Therefore, this mutant appears to have a more general courtship problem that may not be specific to auditory function. The 5P1 mutant appeared to court females as vigorously as did wild-type males (data not shown) but were only half as successful at copulation (Table 2) perhaps because the 5P1 flies were slightly uncoordinated. Two of the other mutations, 5M8 and 5N30, appeared to be associated with significant male sterility that was functional rather than behavioral (Table 2).
Table 2.
Male mutant phenotypes
| Mutation | Copulation* | Fertility | Sperm motility | Map position‡ |
|---|---|---|---|---|
| 40A-G13 (wild-type) | 12/20 | + | + | |
| 5G23 | 11/20 | + | + | |
| 5L3 | 0/20 | −/+† | + | between G13 and c |
| 5M8 | 12/20 | −† | −(thick sperm) | distal to G13 |
| 5N18 | 20/20 | + | + | |
| 5N30 | 16/20 | − | −(degenerate testes) | between G13 and c |
| 5P1 | 6/20 | + | + | near Bl |
Number of males, from a total of 20 tested, that copulated with females within 30 min.
Some 5L3 males eventually did court and produce offspring after several days. One 5M8 male produced offspring after several days.
Map positions of the auditory behavior phenotype are indicated for 5L3, 5M8, and 5N30. 5P1 was mapped on the basis of the sedentary phenotype (see text). In addition, the male sterility of 5M8 was mapped near c, and that of 5N30 was mapped between pr and G13. The G13 insertion site is at 42B on the polytene chromosomes.
Circling Behavior Mutations.
Two of the mutations recovered in this screen, 5G10 and 5N18, showed age-dependent circling behavior, correlated with degeneration in the brain. The first mutant line, 5G10, showed a particularly strong circling phenotype (Fig. 5), and on this basis the mutation was named pirouette (pir). This mutant had strong viability effects such that homozygotes are rare unless grown in uncrowded larval conditions. There was considerable variability in the progression of the adult phenotype. In general, the flies walked rather normally when they eclosed and then progressed through several stages of severity of circling (Fig. 5). Head sections of these flies showed massive degeneration in the brain (Fig. 6). There was a strong correlation between the severity of the behavioral phenotype and the extent of brain degeneration. The degeneration was most apparent in the optic lobes, which coalesced into a dense mass. The other regions of the brain were affected as well, usually apparent as lost cohesiveness between axon tracts and in the cortex. Ommatidial units in the retina dissociated from one another as well. The finding of older flies that show little evidence of degeneration and no behavioral abnormalities exemplified the variability in the phenotype.
Figure 5.
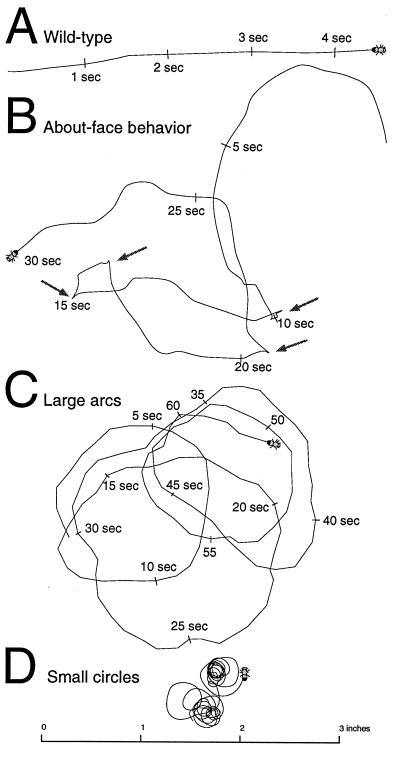
Pirouette adult circling behavior. Walking paths of flies on gridded paper were traced from videotapes. Wild-type flies walked in straight lines (A). (B) The earliest unusual behavior detected in pir5G10 homozygotes was wandering with occasional “about-faces,” 180° turns. With age, pir flies progressed through stages of walking in large arcs or circles usually at 2–5 days old (C) and then smaller and smaller circles at 3–10 days old (D). In A-C, the positions of the flies at regular time points are indicated. The path in D represents 20 revolutions in 1 min. In the final day before death, they no longer walked, and their legs were often seen trembling.
The second mutation that showed adult circling behavior, 5N18, fully complemented the pir chromosome in circling behavior, auditory response, and brain degeneration phenotypes. The circling behavior appeared only in about one–third of 5N18 homozygotes and only after the flies were several weeks old. Brain degeneration was only apparent in those flies that showed circling tendencies and was much milder than in pir, with holes in many regions of the brain, particularly the optic lobes, reminiscent of the vacuolar class of brain morphology mutations (27, 28). The defects in 5N18 flies must have affected regions involved in both the auditory and olfactory pathways and likely the visual pathway as well.
Genetic Mapping.
The auditory behavior phenotype was mapped in three mutants, 5L3, 5M8, and 5N30 to the right arm (Table 2). The 5P1 mutant was somewhat sedentary, often remaining in the bottom of the vial. Preliminary results from mapping this behavior relative to a dominantly marked chromosome indicate that the 5P1 mutation maps near Bl in the proximal region of the left arm. The circling phenotype in 5G10 appeared to map between the G13 insert and the Lrm mutation on the proximal right arm. Further recombination and deficiency mapping suggested that mutations at two loci in this interval may contribute to this phenotype (data not shown). The male sterility and auditory phenotypes of 5M8 mapped to the same broad region whereas those of 5N30 resulted from mutations at different loci (Table 2).
DISCUSSION
The response patterns exhibited by wild-type flies with and without aristae were consistent with the idea prevalent in the literature that the arista is the transformer structure that resonates in the presence of the sound, transferring its vibrations to the articulating stalk of the third segment to stimulate Johnston’s organ sensilla. Thus, removal of the arista prevents capture of the sound energy at low and intermediate sound intensities. However, the sensilla are still intact, and because objects can be made to vibrate at frequencies disparate from their characteristic frequency by increasing the energy in the sound, it is possible that the funiculus can vibrate at the highest sound intensities and thereby assume the role of the transformer. An alternative explanation is that other mechanosensory organs, such as the chordotonal organs in other appendages, or bristles or campaniform sensilla may be activated at this noncharacteristic frequency when enough sound energy is applied.
The auditory behavior described by von Schilcher (13) represents a pathway with the airborne pulse song as a stimulus that triggers male courtship as a response. Mutagenesis of this behavior should allow recovery of mutations that disrupt the morphogenesis or function of the components at any step in this long pathway, from the transformer that converts the acoustic signal into a mechanical one, to the transducer or sensory cell that converts the mechanical signal into an electrochemical one, to the translator, the neuronal circuitry that assimilates the auditory and other sensory information and generates motor signals, to the transactors, the effectors that carry out the behavior.
Such a long pathway provides a large mutagenesis target. This is consistent with our recovery of 15 mutations among ≈400 homozygous viable lines that were recovered in a mutagenesis whereby 60% of the chromosomes were zygotic lethals. One might expect a priori that some of these would be specific to the sensory part of the pathway, others to central processing, others to motor function, and some would be more general, affecting multiple processes. It is important to be able to distinguish among these classes. We have begun classifying these mutations using the audiograms, olfactory tests, and other behavioral phenotypes.
The audiograms distinguished three classes of auditory behavior mutations. The responses of the two major classes were independent of sound intensity, either showing no response or a reduced response at all intensities. These profiles did not provide any information regarding the step that is affected. The differences between the reduced-response mutants and the no-response ones may be explained in some cases by hypomorphic vs. null mutations in integral components of the pathway. In other cases, the difference may lie in the mutations being in ancillary vs. integral steps in the pathway. The final class distinguished by the audiograms, represented by the 5L3 mutant, had a sound intensity-dependent response. The similarity of this response to that of aristaless flies was striking, and taken alone, this phenotype would suggest that 5L3 could be a sensory mutant. However, the poor courtship of females by 5L3 males argues that 5L3 is likely a more general courtship behavior mutant, affecting an intermediate part of the pathway.
The audiogram classes are further subdivided by other criteria. Four of six mutants tested for olfactory behavior showed a normal olfactory response, indicating that these mutants did not have a general defect. One olfactory defective mutant, 5N18, along with the pir mutant 5G10, was also distinguishable by adult circling behavior. We have found no previous report of circling behavior in Drosophila mutants, but the underlying cause appears to be broad neurodegeneration. Perhaps the genetic background in which these mutations were generated is particularly sensitive to the recovery of such mutations, considering findings that the genetic background can strongly affect the expressivity of brain morphology mutations (29).
Further subdivision was achieved by testing whether mutant males will court normal virgin females. Auditory sensory mutants should court well, and more general courtship mutants should court poorly. Four of the six mutants tested courted females at least as much as did the wild type. The low female courtship and copulation rate of the 5L3 mutant males indicates a general courtship behavior defect. Conversely, the 5P1 mutant courted females as vigorously as did the wild type but was only half as successful in copulation, a likely result of reduced coordination and olfaction.
Finally, the most useful test to distinguish sensory mutants from central or motor ones may be to develop an electrophysiological preparation to record from the antennal nerve. This would be useful both for elucidating the information coding mechanisms that mediate recognition of the species-specific parameters of courtship songs and for unraveling with greater precision the specific defects caused by auditory mutations.
Acknowledgments
We are grateful to Anne Lanjuin and Chuck Arnold for generating the mutagenized lines and to Dean Hartley for contributing to the analysis of pir. We thank many colleagues, including Mei-ling Joiner, Leslie Griffith, Jeff Hall, Adriana Villella, John Rosowski, Henry Bennet-Clark, and Maurice Kernan for helpful discussions and Jelveh Ghazizadeh and Mark Neilson for computer programming assistance. D.F.E. was supported by funds from the Massachusetts Eye and Ear Infirmary, Howard Hughes Medical Institute, and the Human Science Frontier Program Organization. N.P. is an Investigator and G.M.D. was an Assistant Investigator of the Howard Hughes Medical Institute.
References
- 1.García-Añoveros J, Corey D P. Annu Rev Neurosci. 1997;20:567–594. doi: 10.1146/annurev.neuro.20.1.567. [DOI] [PubMed] [Google Scholar]
- 2.Kernan M, Cowan D, Zuker C. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 3.Gillespie P G. Curr Opin Neurobiol. 1995;5:449–455. doi: 10.1016/0959-4388(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 4.Steel K P, Brown S D M. Curr Opin Neurobiol. 1996;6:520–525. doi: 10.1016/s0959-4388(96)80059-6. [DOI] [PubMed] [Google Scholar]
- 5.Hall J C. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 6.Shorey H H. Science. 1962;137:677–678. doi: 10.1126/science.137.3531.677. [DOI] [PubMed] [Google Scholar]
- 7.Ewing A W, Bennet-Clark H C. Behavior. 1968;31:288–301. [Google Scholar]
- 8.Bennet-Clark H C, Ewing A W. Anim Behav. 1969;17:755–759. [Google Scholar]
- 9.Kyriacou C P, Hall J C. Anim Behav. 1982;30:794–801. [Google Scholar]
- 10.Kyriacou C P, Hall J C. Anim Behav. 1989;37:850–859. [Google Scholar]
- 11.Crossley S A. Anim Behav. 1988;36:1098–1109. [Google Scholar]
- 12.Ewing A W. Anim Behav. 1988;36:1091–1097. [Google Scholar]
- 13.von Schilcher F. Anim Behav. 1976;24:18–26. [Google Scholar]
- 14.von Schilcher F. Anim Behav. 1976;24:622–625. [Google Scholar]
- 15.Paillette M, Ikeda H, Jallon J-M. Bioacoustics. 1991;3:247–254. [Google Scholar]
- 16.Johnston, C. (1855) Q. J. Microscop. Sci. 3, Old Series, 97–102.
- 17.Petit C. Bull Biol. 1958;92:248–327. [Google Scholar]
- 18.Manning A. Science. 1967;158:136–137. doi: 10.1126/science.158.3797.136. [DOI] [PubMed] [Google Scholar]
- 19.Burnet B, Connolly K, Dennis L. Anim Behav. 1971;19:409–415. doi: 10.1016/s0003-3472(71)80025-8. [DOI] [PubMed] [Google Scholar]
- 20.Ewing A W. Physiol Entomol. 1978;3:33–36. [Google Scholar]
- 21.Uga S, Kuwabara M. J Electron Microsc. 1965;14:173–181. [PubMed] [Google Scholar]
- 22.Jarman A P, Grau Y, Jan L Y, Jan Y N. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-w. [DOI] [PubMed] [Google Scholar]
- 23.Chou T-B, Perrimon N. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis E B, Bacher F. Dros Inform Serv. 1968;43:193. [Google Scholar]
- 25.Woodard C, Huang T, Sun H, Helfand S L, Carlson J. Genetics. 1989;123:315–326. doi: 10.1093/genetics/123.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennet-Clark H C. Nature (London) 1971;234:255–259. [Google Scholar]
- 27.Heisenberg M, Böhl K. Z Naturforsch, C. 1979;34:143–147. [Google Scholar]
- 28.Buchanan R L, Benzer S. Neuron. 1993;10:839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- 29.de Belle J S, Heisenberg M. Proc Natl Acad Sci USA. 1996;93:9875–9880. doi: 10.1073/pnas.93.18.9875. [DOI] [PMC free article] [PubMed] [Google Scholar]