Abstract
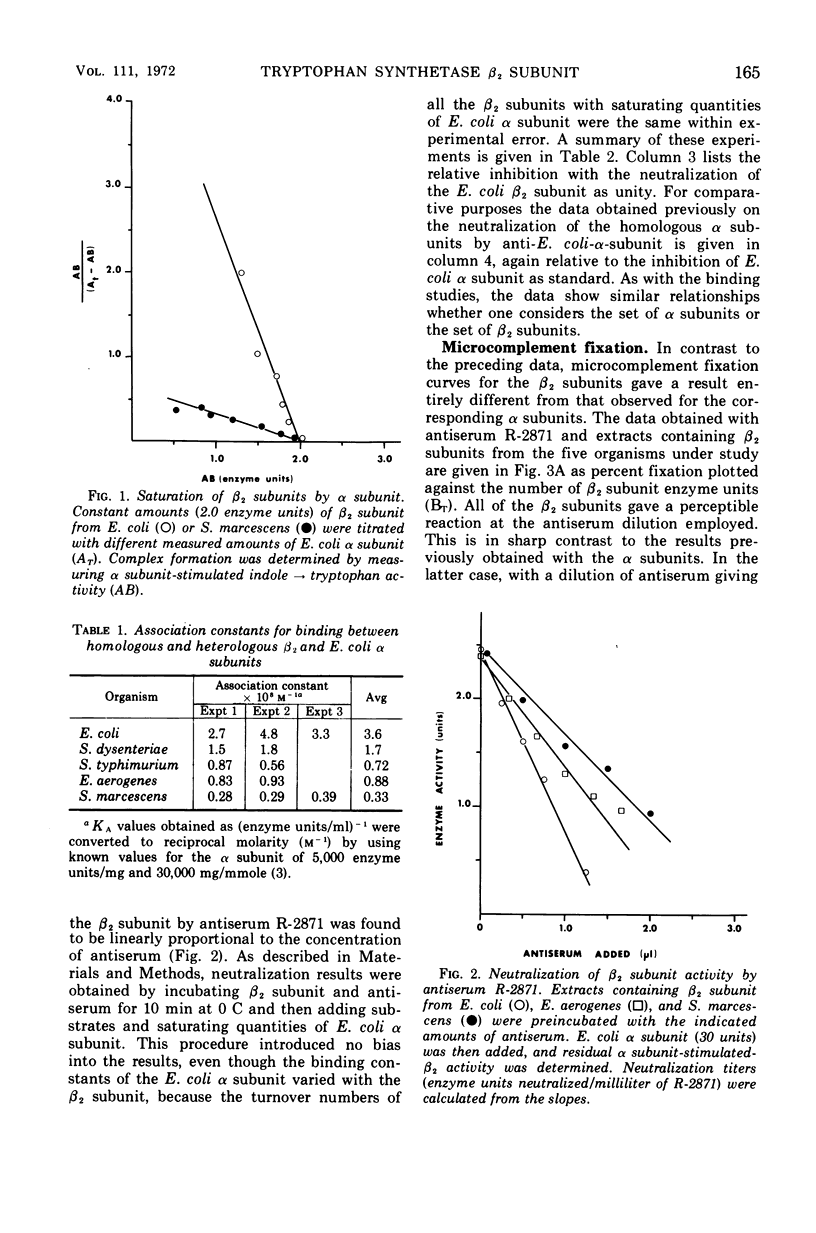
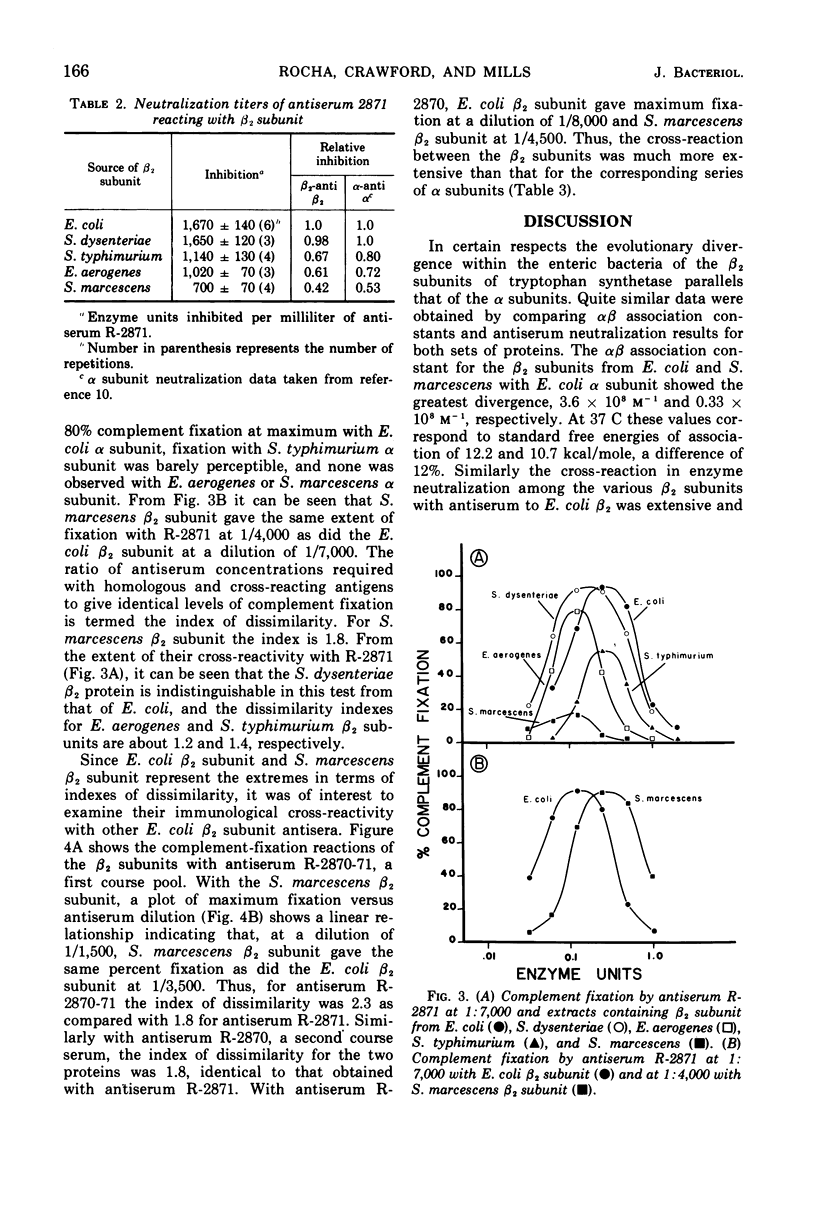
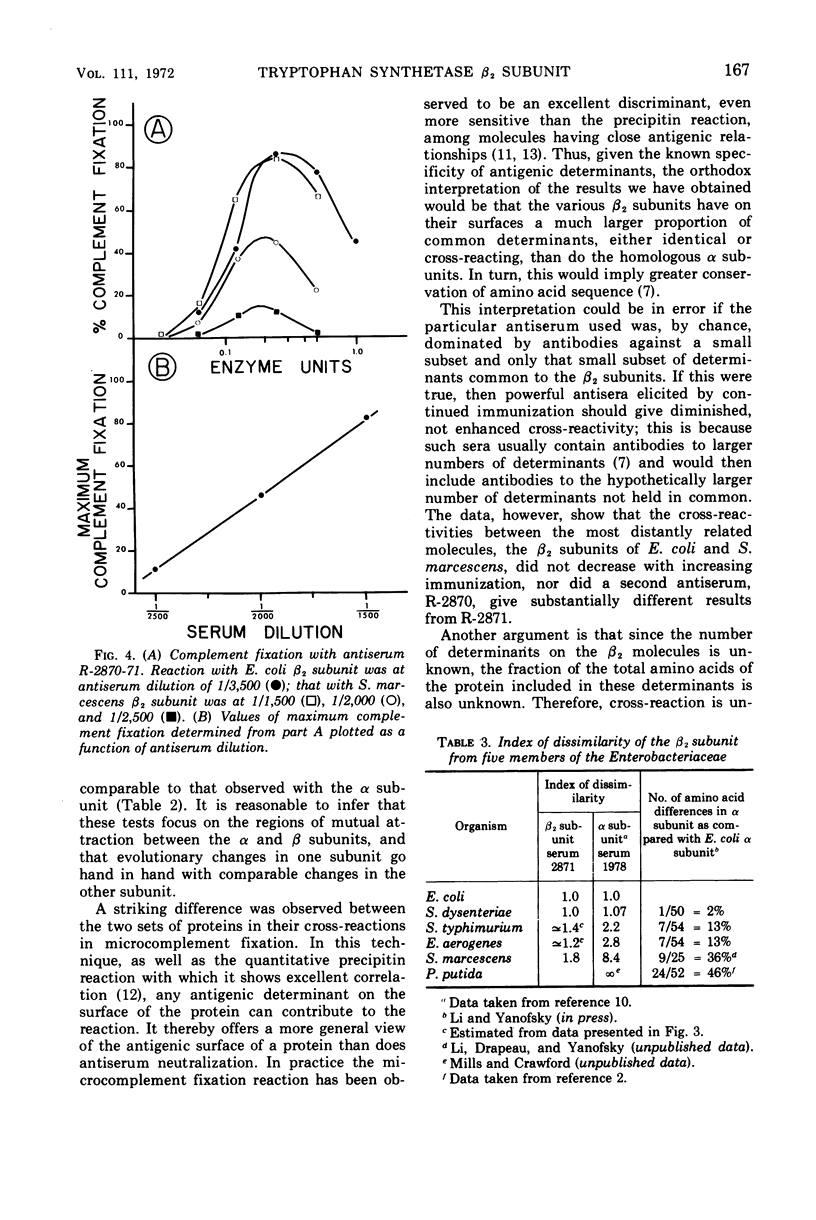
The β2 subunits of tryptophan synthetase, formula α2β2, from Escherichia coli, Shigella dysenteriae, Enterobacter aerogenes, Salmonella typhimurium, and Serratia marcescens were compared by three criteria. (i) αβ association constants for the various β2 subunits and E. coli α subunit varied between 3.6 × 108m−1 for E. coli and 0.33 × 108m−1 for S. marcescens; values for the other organisms were intermediate. (ii) Antiserum neutralization of the β2 subunit enzyme activity using anti-E. coli β2 serum showed significant cross-reaction among the organisms (E. coli, 1.0; S. dysenteriae, 0.98; S. typhimurium, 0.67; E. aerogenes, 0.61; S. marcescens, 0.42). (iii) Quantitative microcomplement fixation showed E. coli β2 and S. marcescens β2 subunits to have an index of dissimilarity of 1.8 while the other organisms had intermediate indexes. Similar complement fixation data were obtained with antisera from separate rabbits and from first course and boost sera. These findings suggest that the general surface structure and the respective α subunit binding site of the β2 subunits from these Enterobacteriaceae have been strongly conserved.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford I. P., Yanofsky C. ON THE SEPARATION OF THE TRYPTOPHAN SYNTHETASE OF ESCHERICHIA COLI INTO TWO PROTEIN COMPONENTS. Proc Natl Acad Sci U S A. 1958 Dec 15;44(12):1161–1170. doi: 10.1073/pnas.44.12.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. Pseudomonas putida tryptophan synthetase: partial sequence of the subunit. J Bacteriol. 1971 Oct;108(1):248–253. doi: 10.1128/jb.108.1.248-253.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Association of the alpha and beta-2 subunits of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):980–990. [PubMed] [Google Scholar]
- Dickerson R. E., Takano T., Eisenberg D., Kallai O. B., Samson L., Cooper A., Margoliash E. Ferricytochrome c. I. General features of the horse and bonito proteins at 2.8 A resolution. J Biol Chem. 1971 Mar 10;246(5):1511–1535. [PubMed] [Google Scholar]
- HENNING U., HELINSKI D. R., CHAO F. C., YANOFSKY C. The A protein of the tryptophan synthetase of Escherichia coli. Purification, crystallization, and composition studies. J Biol Chem. 1962 May;237:1523–1530. [PubMed] [Google Scholar]
- Hathaway G. M., Crawford I. P. Studies on the association of beta-chain monomers of Escherichia coli tryptophan synthetase. Biochemistry. 1970 Apr 14;9(8):1801–1808. doi: 10.1021/bi00810a020. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical comparisons of mutant and wild-type alpha-subunits of tryptophan synthetase. Arch Biochem Biophys. 1968 Sep 20;127(1):7–16. doi: 10.1016/0003-9861(68)90194-x. [DOI] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. II. Comparison of precipitin and micro-complement fixation results. J Biol Chem. 1971 Nov 25;246(22):7010–7017. [PubMed] [Google Scholar]
- REICHLIN M., HAY M., LEVINE L. ANTIBODIES TO HUMAN A1 HEMOGLOBIN AND THEIR REACTION WITH A2, S, C, AND H HEMOGLOBINS. Immunochemistry. 1964 Apr;1:21–30. doi: 10.1016/0019-2791(64)90052-7. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Wilson D. A., Crawford I. P. Purification and properties of the B component of Escherichia coli tryptophan synthetase. J Biol Chem. 1965 Dec;240(12):4801–4808. [PubMed] [Google Scholar]


