Abstract
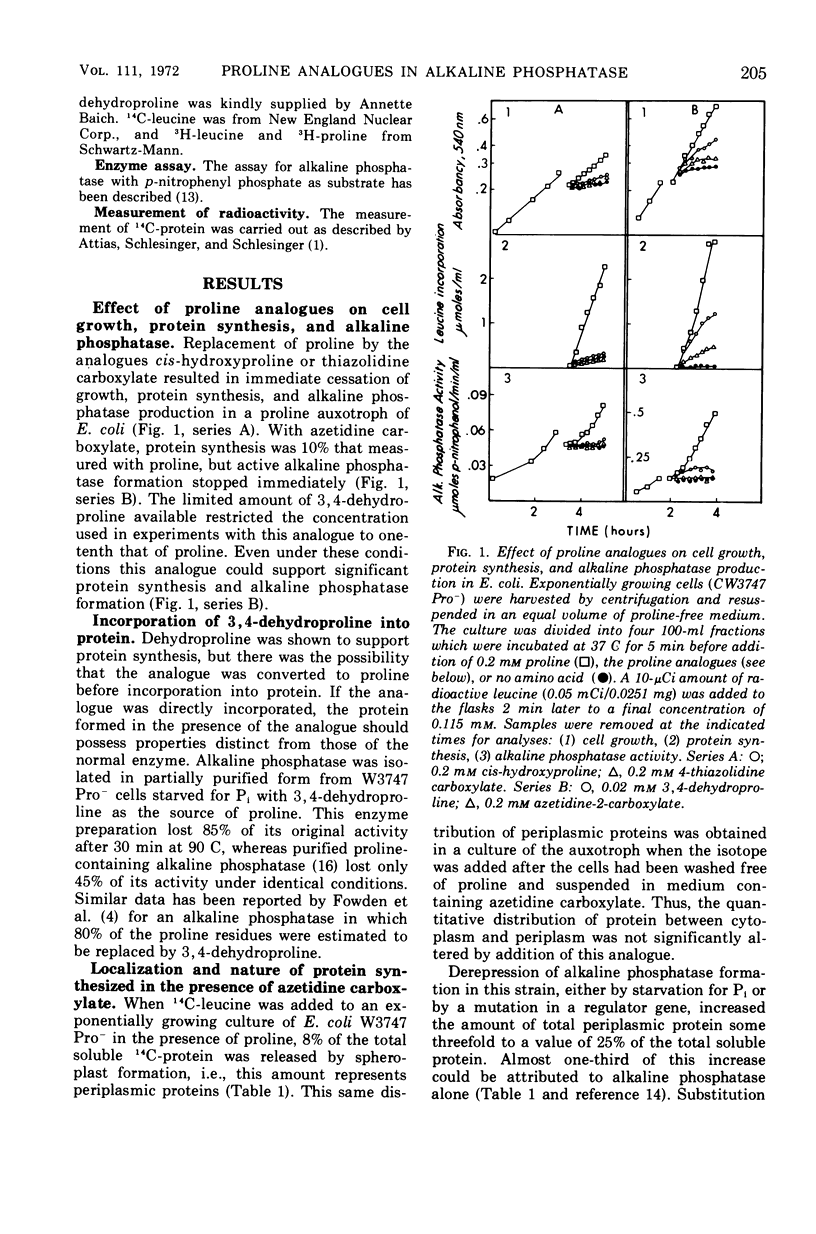
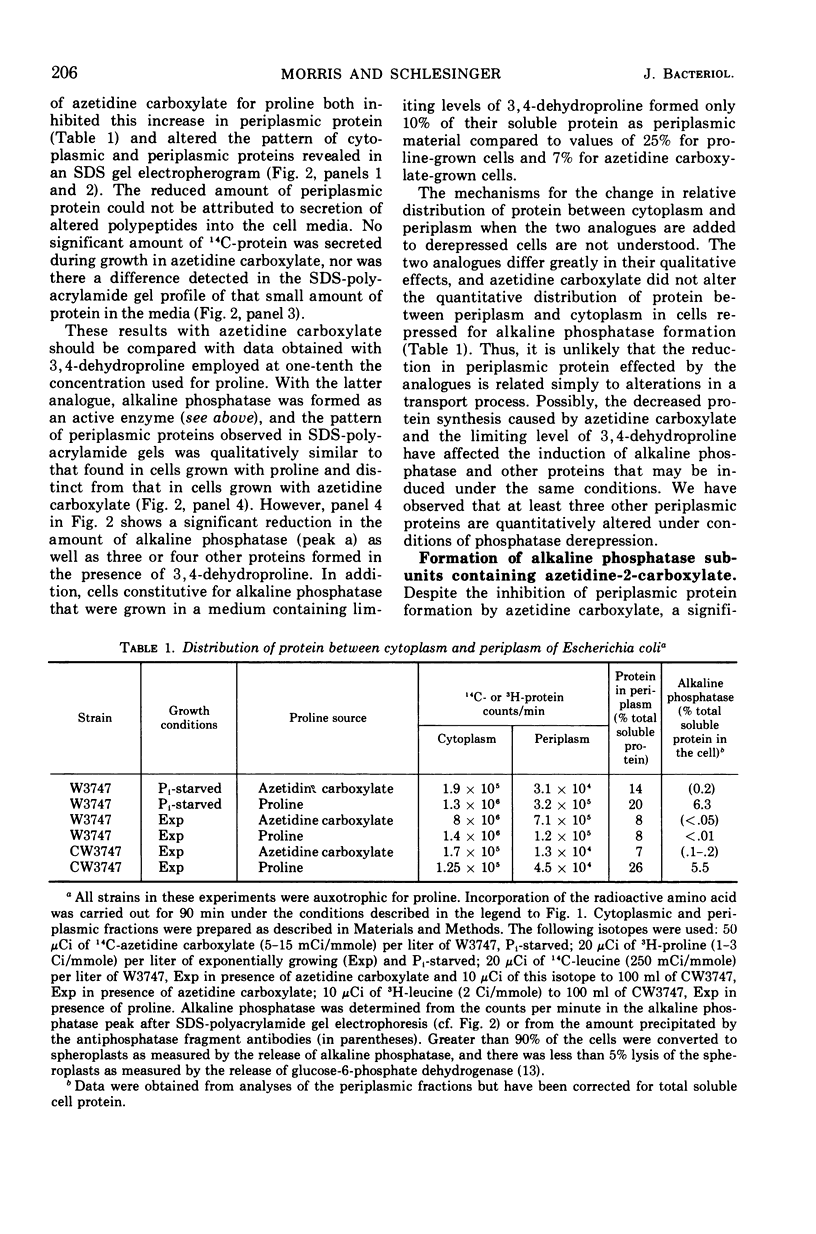
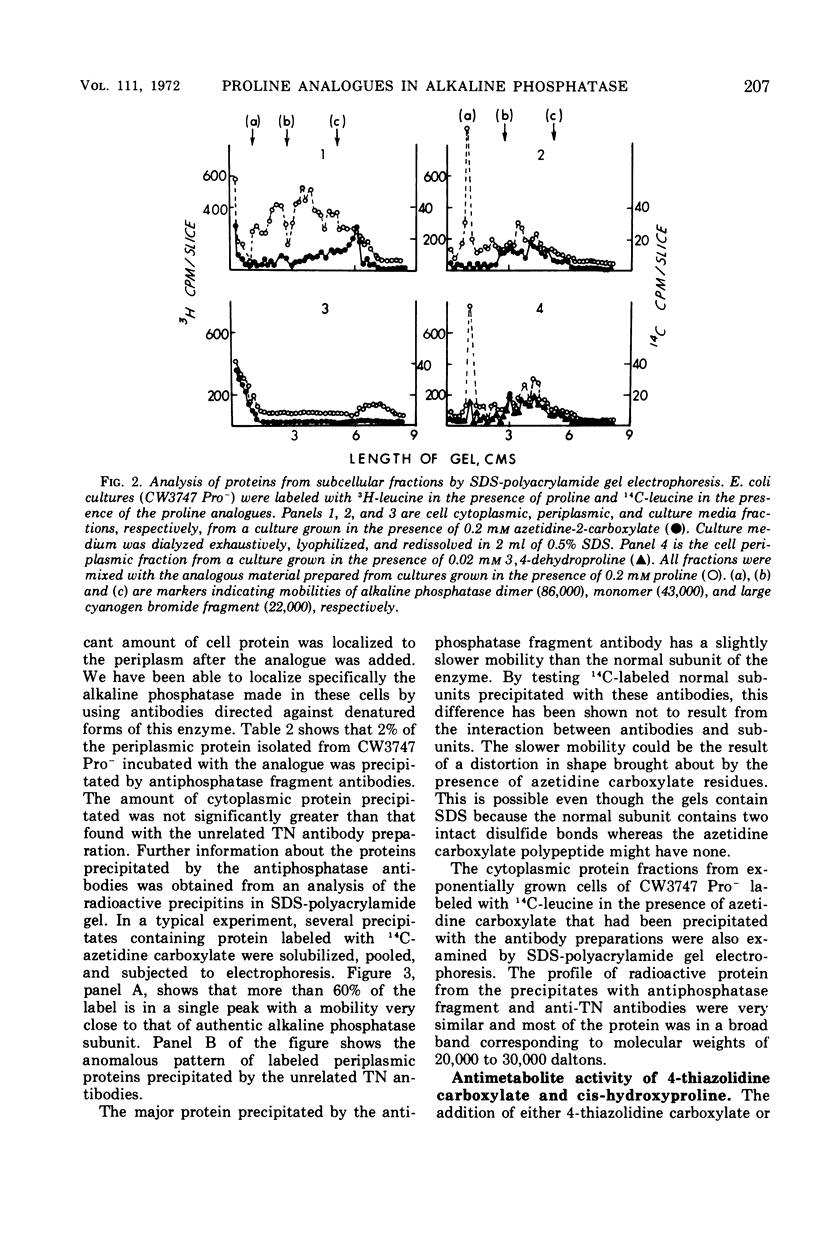
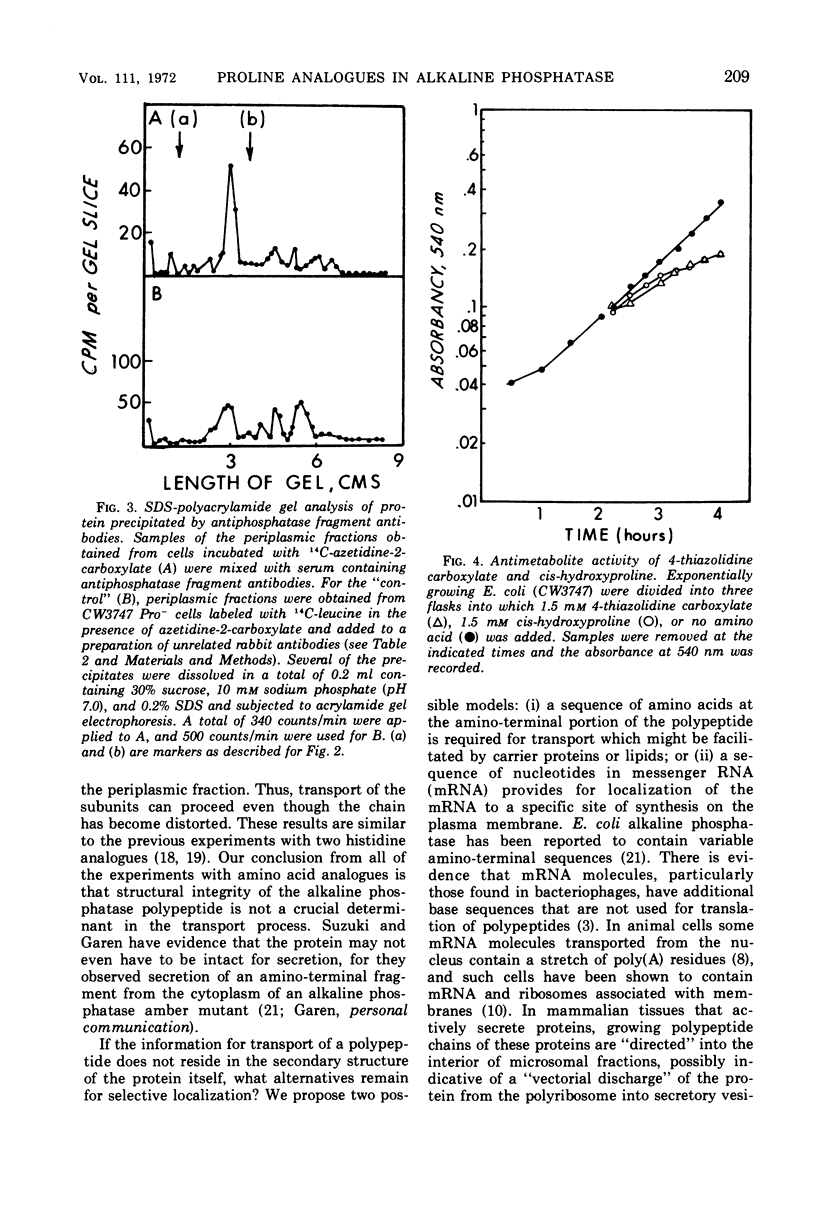
Two of the four proline analogues tested for their effect on the formation and activity of Escherichia coli alkaline phosphatase were able to substitute for proline in protein synthesis in a proline auxotroph. One of these, 3,4-dehydroproline, effectively replaced proline and led to formation of an active enzyme under conditions where no proline was present in the polypeptides. Substitution of azetidine-2-carboxylate for proline prevented active enzyme formation, producing instead altered monomeric forms of the alkaline phosphatase. These were detected with antibodies specific to denatured forms of the enzyme, and they were also characterized, together with cellular proteins, by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Alkaline phosphatase, as well as several other proteins, is localized exterior to the bacterial cell cytoplasm in the periplasmic space. In the presence of azetidine-2-carboxylate, a substantial number of these periplasmic proteins retain their specific site of localization, and the denatured subunits of alkaline phosphatase were only detected in the periplasmic fraction of the cell. Thus, secretion of these proteins does not appear to require a high degree of specificity in the native structure of the polypeptide chain. The analogues 4-allohydroxyproline and 4-thiazolidine carboxylate were unable to substitute for proline in protein synthesis but they inhibited growth of E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attias J., Schlesinger M. J., Schlesinger S. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. IV. Substitution of canavanine for arginine. J Biol Chem. 1969 Jul 25;244(14):3810–3817. [PubMed] [Google Scholar]
- Berman H. M., McGandy E. L., Burgner J. W., 2nd, VanEtten R. L. The crystal and molecular structure of L-azetidine-2-carboxylic acid. A naturally occurring homolog of proline. J Am Chem Soc. 1969 Oct 22;91(22):6177–6182. doi: 10.1021/ja01050a044. [DOI] [PubMed] [Google Scholar]
- FOWDEN L., NEALE S., TRISTRAM H. EFFECT OF 3,4-DEHYDRO-DL-PROLINE ON GROWTH AND PROTEIN SYNTHESIS. Nature. 1963 Jul 6;199:35–38. doi: 10.1038/199035a0. [DOI] [PubMed] [Google Scholar]
- Fowden L., Lewis D., Tristram H. Toxic amino acids: their action as antimetabolites. Adv Enzymol Relat Areas Mol Biol. 1967;29:89–163. doi: 10.1002/9780470122747.ch3. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. II. Factors affecting secretion. J Biol Chem. 1971 Mar 25;246(6):1566–1574. [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C. M., Sabatini D. D. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci U S A. 1966 Aug;56(2):608–615. doi: 10.1073/pnas.56.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M., Penman S. Membrane-associated protein synthesis of mammalian cells. I. The two classes of membrane-associated ribosomes. J Mol Biol. 1971 Jul 28;59(2):227–241. doi: 10.1016/0022-2836(71)90048-9. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Formation of a defective alkaline phosphatase subunit by a mutant of Escherichia coli. J Biol Chem. 1967 Apr 10;242(7):1604–1611. [PubMed] [Google Scholar]
- Schlesinger M. J., Olsen R. A new, simple, rapid procedure for purification of Escherichia coli alkaline phosphatase. Anal Biochem. 1970 Jul;36(1):86–90. doi: 10.1016/0003-2697(70)90334-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. The reversible dissociation of the alkaline phosphatase of Escherichia coli. 3. Properties of antibodies directed against the subunit. J Biol Chem. 1967 Apr 10;242(7):1599–1603. [PubMed] [Google Scholar]
- Schlesinger M. J. The reversible dissociation of the alkaline phosphatase of Escherichia coli. II. Properties of the subunit. J Biol Chem. 1965 Nov;240(11):4293–4298. [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. 3. Substitution of 2-methylhistidine for histidine. J Biol Chem. 1969 Jul 25;244(14):3803–3809. [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. I. Substitution of triazolealanine for histidine. J Biol Chem. 1967 Jul 25;242(14):3369–3372. [PubMed] [Google Scholar]
- Schlesinger S. The effect of amino acid analogues on alkaline phosphatase. Formation in Escherichia coli K-12. II. Replacement of tryptophan by azatryptophan and by tryptazan. J Biol Chem. 1968 Jul 25;243(14):3877–3883. [PubMed] [Google Scholar]
- Suzuki T., Garen A. Fragments of alkaline phosphatase from nonsense mutants. I. Isolation and characterization of fragments from amber and ochre mutants. J Mol Biol. 1969 Nov 14;45(3):549–566. doi: 10.1016/0022-2836(69)90312-x. [DOI] [PubMed] [Google Scholar]
- Unger L., DeMoss R. D. Action of a proline analogue, l-thiazolidine-4-carboxylic acid, in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1556–1563. doi: 10.1128/jb.91.4.1556-1563.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]


