Abstract
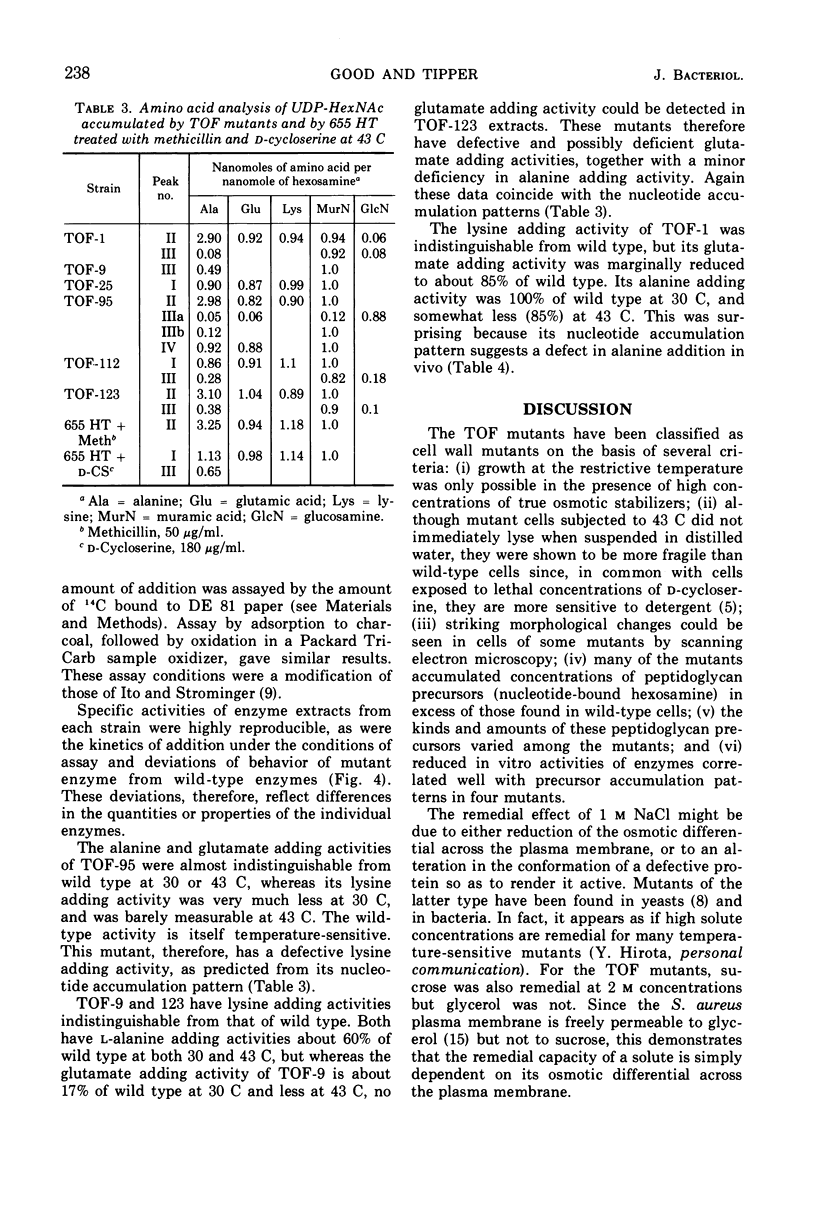
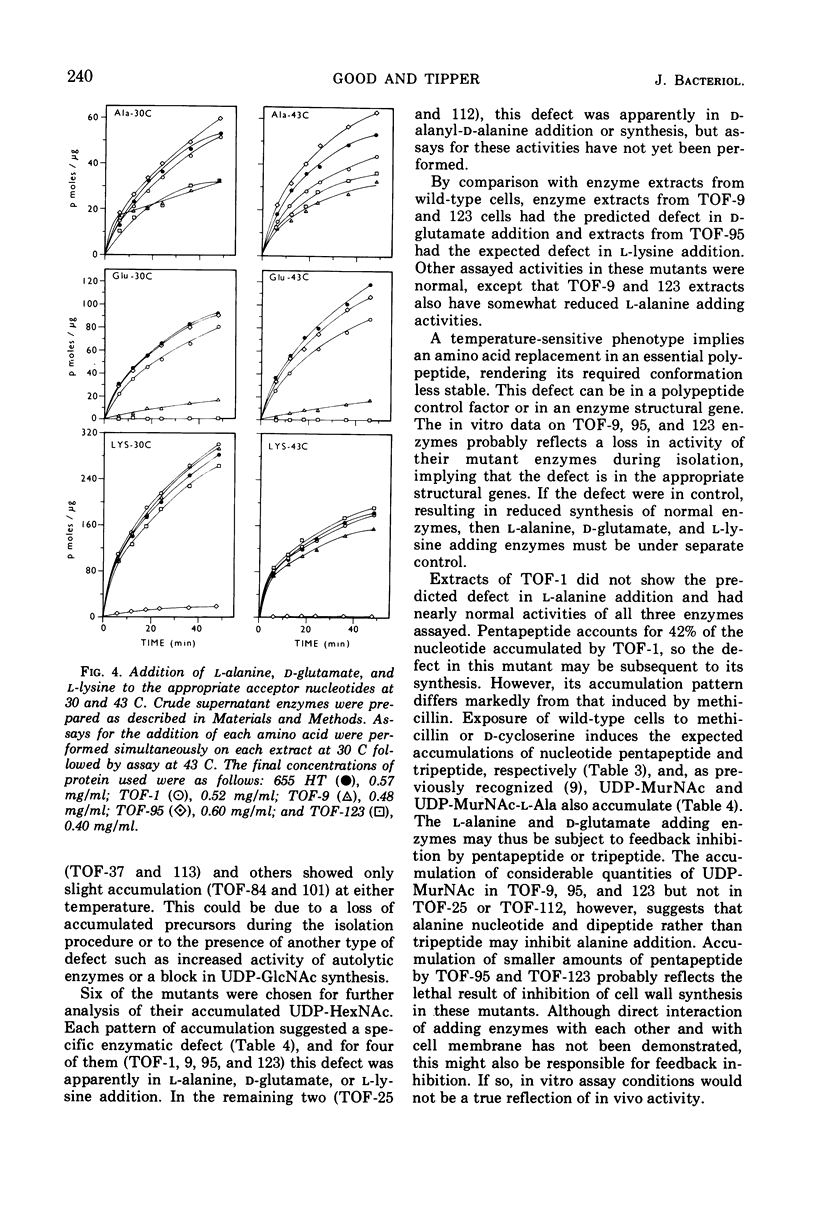
Temperature-sensitive (ts) mutants of Staphylococcus aureus with defective cell wall biosynthesis have been differentiated from other ts mutants by their ability to grow at the restrictive temperature (43 C) in the presence of 1 m NaCl. Under all conditions they possess normal colonial and cellular morphology at the level of resolution of the light microscope and are, therefore, not protoplasts. However, differences between mutant and wild-type cells can be seen by scanning electron microscopy. Many of the mutants contained concentrations of nucleotide precursors of peptidoglycan synthesis in excess of those present in wild-type cells, at both 30 and 43 C. The types of peptidoglycan precursors accumulated by six of the mutants have been determined, and specific enzymatic defects in three of these have been identified.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassel J., Douglas H. C. Relationship between solute permeability and osmotic remediability in a galactose-negative strain of Saccharomyces cerevisiae. J Bacteriol. 1970 Nov;104(2):707–711. doi: 10.1128/jb.104.2.707-711.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Young F. E. Regulation of the bacterial cell wall: isolation and characterization of peptidoglycan mutants of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):220–230. doi: 10.1128/jb.111.1.220-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Carleton J., Watanakunakorn C., Klainer A. S., Hamburger M. Scanning-beam electron microscopy of cell wall-defective staphylococci. Infect Immun. 1970 Oct;2(4):504–515. doi: 10.1128/iai.2.4.504-515.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C. M., Pattee P. A. Temperature-Sensitive Osmotically Fragile Mutants of Staphylococcus aureus. J Bacteriol. 1970 Dec;104(3):1401–1403. doi: 10.1128/jb.104.3.1401-1403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman H., Pattee P. A. Synchronous growth of Staphylococcus aureus induced by amino acid and thymine starvation. Can J Microbiol. 1970 Dec;16(12):1371–1374. doi: 10.1139/m70-228. [DOI] [PubMed] [Google Scholar]
- HAWTHORNE D. C., FRIIS J. OSMOTIC-REMEDIAL MUTANTS. A NEW CLASSIFICATION FOR NUTRITIONAL MUTANTS IN YEAST. Genetics. 1964 Nov;50:829–839. doi: 10.1093/genetics/50.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger M., Carleton J. Staphylococcal spheroplasts and L colonies. I. Population curves of vegetative staphylococci and spheroplasts in methicillin-containing broth over long periods. J Infect Dis. 1966 Apr;116(2):221–230. doi: 10.1093/infdis/116.2.221. [DOI] [PubMed] [Google Scholar]
- KLOSS W. E., PATTEE P. A. TRANSDUCTION ANALYSIS OF THE HISTIDINE REGION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1965 May;39:195–207. doi: 10.1099/00221287-39-2-195. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg E. J., De Haas-Menger L., Ruyters W. H. Murein synthesis and identification of cell wall precursors of temperature-sensitive lysis mutants of Escherichia coli. J Bacteriol. 1972 Jan;109(1):326–335. doi: 10.1128/jb.109.1.326-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. Autolytic release and osmotic properties of protoplasts from Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):184–194. doi: 10.1099/00221287-16-1-184. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Apirion D., Schlessinger D. Selection of sucrose-dependent Escherichia coli to obtain envelope mutants and fragile cultures. Science. 1966 Aug 19;153(3738):892–894. doi: 10.1126/science.153.3738.892. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Ishimoto N., Anderson J. S., Strominger J. L. Enzymatic synthesis and immunochemistry of alpha- and beta-N-acetylglucosaminylribitol linkages in teichoic acids from several strains of Staphylococcus aureus. J Biol Chem. 1966 Feb 10;241(3):651–658. [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. Cell wall or membrane mutants of Bacillus subtilis and Bacillus licheniformis with grossly deformed morphology. Nature. 1968 Jul 20;219(5151):285–288. doi: 10.1038/219285a0. [DOI] [PubMed] [Google Scholar]
- STROMINGER J. L. Microbial uridine-5'-pyrophosphate N-acetylamino sugar compounds. I. Biology of the penicillin-induced accumulation. J Biol Chem. 1957 Jan;224(1):509–523. [PubMed] [Google Scholar]
- Tipper D. J., Pratt I. Cell wall polymers of Bacillus sphaericus 9602. II. Synthesis of the first enzyme unique to cortex synthesis during sporulation. J Bacteriol. 1970 Aug;103(2):305–317. doi: 10.1128/jb.103.2.305-317.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]




