Abstract
Pregnenolone sulfate (PREG S) is synthesized in the nervous system and is a major neurosteroid in the rat brain. Its concentrations were measured in the hippocampus and other brain areas of single adult and aged (22–24 month-old) male Sprague–Dawley rats. Significantly lower levels were found in aged rats, although the values were widely scattered and reached, in about half the animals, the same range as those of young ones. The spatial memory performances of aged rats were investigated in two different spatial memory tasks, the Morris water maze and Y-maze. Performances in both tests were significantly correlated and, accompanied by appropriate controls, likely evaluated genuine memory function. Importantly, individual hippocampal PREG S and distance to reach the platform in the water maze were linked by a significant correlation, i.e., those rats with lower memory deficit had the highest PREG S levels, whereas no relationship was found with the PREG S content in other brain areas (amygdala, prefrontal cortex, parietal cortex, striatum). Moreover, the memory deficit of cognitively impaired aged rats was transiently corrected after either intraperitoneal or bilateral intrahippocampal injection of PREG S. PREG S is both a γ-aminobutyric acid antagonist and a positive allosteric modulator at the N-methyl-d-aspartate receptor, and may reinforce neurotransmitter system(s) that decline with age. Indeed, intracerebroventricular injection of PREG S was shown to stimulate acetylcholine release in the adult rat hippocampus. In conclusion, it is proposed that the hippocampal content of PREG S plays a physiological role in preserving and/or enhancing cognitive abilities in old animals, possibly via an interaction with central cholinergic systems. Thus, neurosteroids should be further studied in the context of prevention and/or treatment of age-related memory disorders.
The reports of higher concentrations of certain steroids in brain than blood and of their accumulation in brain independently of adrenal and gonadal sources led to the discovery of steroid biosynthetic pathways in the central nervous system. As a result, the term “neurosteroids” was proposed, referring to steroids synthesized in the brain, either de novo from cholesterol or by in situ metabolism of blood-borne precursors (1). Several neurosteroids, apart from their effects on anxiety and convulsions (2), influence cognitive functions and particularly memory processes (3–11). Systemic or intra-cerebral administration of pregnenolone (PREG) or its sulfate ester (PREG S) enhances memory in rodents by increasing the animal’s natural performance, or by antagonizing pharmacologically induced amnesia (5–8, 10, 11). However, in the majority of these studies, the animal, usually a rat or a mouse, must avoid electrical footshocks (active or passive avoidance), or search for a food reward after food deprivation. The suitability of these procedures for evaluating specific memory performance has been questioned (12), since they may induce alterations of the motivational or emotional status of the animals. Moreover, the effects of the administration of steroids on their cerebral concentrations have seldom been evaluated (8, 13). Thus, although pharmacological effects have been described, a direct relationship between brain levels and performance have yet to be established, casting doubt on the physiological significance of these pharmacological studies (14).
Attention is now focused on the physiological roles of neurosteroids. The alone example still reported is the role of progesterone synthesized by Schwann cells in myelin synthesis following nerve injury (15). The suggestion of neurosteroid-induced memory improvements in aged mice (3) led us to investigate the physiological role of PREG S in relationship with age-related cognitive impairments. In humans and animals, cognitive abilities exhibit a natural decline with age, although with considerable interindividual differences (16), which we have exploited in the search for relationships between the performance of each aged animal and PREG S content in several areas of its brain, particularly in the hippocampus. Indeed, this structure is selectively sensitive to aging and is known to be involved in cognitive functions such as learning and spatial orientation (17, 18). Once such correlation was established, a cause to effect relationship was investigated: the memory disturbance of impaired aged rats was indeed corrected by increasing brain PREG S concentration. Finally, a neurotransmitter system likely involved in the cognitive effects of PREG S was assessed, the steroid was injected intracerebroventricularly (i.c.v.) and its effect on the extracellular concentrations of acetylcholine (ACh) in the hippocampus was determined.
MATERIALS AND METHODS
Animals and Housing Conditions.
Male Sprague–Dawley rats (Iffa Credo) were used in all experiments. Animals had ad libitum access to food and water and were housed individually. The light-dark cycle (lights were on from 6 am to 8 pm), temperature (22°C), and humidity (60%) were kept constant in the animal house. All experiments were carried out in accordance with the Helsinki 1975 declaration on animal welfare.
RIA of PREG S.
Animals were sacrificed by decapitation and the brain regions were rapidly dissected and frozen on dry ice. Tissues were stored at −80°C until the measurement of PREG S. Extracts of brain homogenates were prepared by a differential extraction procedure that separates sulfate esters from fatty acid esters and unconjugated steroids. The water phase containing steroid sulfates was solvolysed. The steroids recovered from the solvolysed fraction were purified by chromatography on a C18 microcolumn, then PREG was measured by a specific RIA (19).
Behavioral Testing.
The spatial memory performances of aged rats were investigated in two different memory tasks, the water maze (20) and the Y-maze (21). The Morris water maze was a circular swimming pool (180 cm diameter × 60 cm high) that was filled with opaque water at 22 ± 1°C. The testing room was highly illuminated (350 lx on the liquid surface). Spatial cues were placed in the room, and remained in fixed positions throughout the experiment. The pool was arbitrarily divided into four quadrants. During the behavioral testing, animals were required to locate a hidden submerged platform (1.5 cm below the surface) in the same quadrant throughout the task using only the available spatial cues. The animals were given four trials per day for 13 days and the starting positions changed over trials. Each trial began with the animal in the pool facing the sidewalls and ended either after 90 sec of swimming or after the animal had found the platform; in either case the rat remained on the platform for 20 sec between each trial. On the four last days all animals reached the platform within 90 sec and the memory performance was measured by the distance to escape onto the platform rather than escape latency because the former is less dependent on the rats’ swim speed (22). To control the animal’s ability to perform the motor and visual demands of the task, rats were then trained for four trials per day over 3 days with a visible platform (2 cm above the liquid surface). Although the water maze has been widely used for the characterization of cognitive decline in aged rats, it can be considered as an anxiogenic test (review in ref. 23). For this reason and also for practical purposes, we used a two-trial spatial memory task involving spontaneous exploratory behavior that minimizes emotional activation (21, 24). It used a Y-maze with three identical arms (50 cm long, 16 cm wide and 32 cm high) illuminated by dim light (70 lx). Each arm was equipped with two infrared beams, one at each end of the arm. The floor of the maze was covered with rat odor-saturated sawdust and between each session, the sawdust was mixed to eliminate olfactory cues. Visual cues were placed in the testing room and kept constant during the behavioral testing sessions. The test consisted of two trials separated by a time-interval (intertrial interval, ITI). In the first trial (acquisition trial), one arm was closed and animals were allowed to visit the two other arms for 10 min. During the inter-trial interval, rats were housed in their home-cages located in a room other than the test room. In the second trial (retention trial), animals had free access to the three arms, and were again allowed to explore the maze for 10 min. The number of visits in the novel arm (previously closed in the first trial) was calculated as a percentage of the total number of visits in all three arms during the first 2 min of the second trial. This time corresponds to the maximal exploratory activity in the novel arm, which declines thereafter (21). The percentage values were compared with a random level for visits to the three arms, i.e., 33%. Two different inter-trial intervals were used. The memory performance was measured with a 6 h ITI, whereas a 1 min ITI was employed to control the spontaneous novelty exploration of the rats when no retention is required.
Administration of PREG S.
Several groups of rats (to be described below) were treated with either PREG S or vehicle. PREG S was injected either i.p. or directly into the hippocampi or in lateral cerebral ventricle. Large amounts of steroid were required for the i.p. injections because it hardly crosses the blood brain barrier. Based on previous attempts, the amount of PREG S injected in aged rats (24 months-old) was 47.5 mg/4 ml per kg. Control aged animals received 4 ml/kg of 0.9% NaCl. PREG S was also injected bilaterally into the dorsal hippocampus. The aged rats (22-month-old) were submitted to implantation of chronic indwelling cannulae (stainless steel, 23 gauge, 7 mm long). They were inserted 1 mm above the dorsal hippocampus at the following coordinates [according to Paxinos and Watson (25)]: from the bregma, anteroposteriorly (A) −4.3 mm, laterally (L), ±2.4 mm, and ventrally (V) from the skull surface, −2.6 mm. When needed, bilateral infusions of either PREG S (5 ng) or saline were made directly into the dorsal hippocampus. Infusions (0.5 μl/90 sec) were made through 30-gauge needles extending 1 mm beyond the ventral tip of the cannulae. The i.c.v. administration of PREG S was performed in adult rats (3-month-old). Animals were submitted to implantation of a guide cannula (23 gauge, 10.00 mm long) into the cortex above the lateral ventricle (A, 0 mm, L, 1.3 mm, from the bregma and from dura V, −2.5 mm, with incisor bar set at +5.0 mm) such that the 30-gauge injection cannula used during experiments, which extended 1.5 mm beyond the guide, would cross the corpus callosum to reach the ventricle. The cannulae for intrahippocampal or i.c.v. injections were connected with polyethylene tubing to a pump-driven microsyringe. After completion of the experiments, injection sites were checked histologically on 100-μm thick thionin-stained coronal sections. Only the animals with correctly placed injection cannulae were included in the statistical analyses of data.
Microdialysis and Assay of ACh.
Under sodium pentobarbital anesthesia (50 mg/kg i.p.) each rat was implanted with one transverse dialysis probe into the dorsal hippocampus. The coordinates relative to bregma were: A, −4.3 mm; V, −3.3 mm. The dialysis probes were made of acrilonitrile-sodium methallyl sulfonate fibre (inner diameter = 220 μm, outer diameter = 310 μm, molecular mass cutoff >60,000 Da; Filtral AN69, Hospal, provided by Vancouver Hospital, Canada) and had an active surface length of 6.8 mm. After a 48-h postoperative period, brain microdialysis was performed as described (26, 27). During experiments each rat was housed in a Plexiglas cage (31 × 32 × 35), to which it had been habituated overnight, with free access to food and water. Brain microdialysis was performed with a fully automated on-line system (see ref. 28 for detailed description). The dialysis probe was perfused at 5 μl/min, controlled by a syringe pump (BAS, Cheshire, UK). The perfusion fluid was a modified artificial cerebrospinal fluid (125 mM NaCl/3 mM KCl/1.3 mM CaCl2/1.0 mM MgCl2/23 mM NaHCO3 buffered at pH 7.4). To recover detectable dialysate concentrations of ACh, a reversible cholinesterase inhibitor (neostigmine bromide, 0.1 μM; Sigma) was included in the perfusion solution.
ACh was assayed by HPLC with electrochemical detection in conjunction with an enzyme reactor (26, 29). ACh and choline were separated on a reverse phase column (75 × 2.1 mm) pretreated with lauryl sulfate. The eluate from this analytical column then passed through an enzyme reactor (10 × 2.1 mm) containing acetylcholinesterase (EC 3.1.1.7; Sigma, type VI-S) and choline oxidase (1.1.3.17; Sigma), covalently bound to glutaraldehyde-activated Lichrosorb NH2 (10 μm; Merck). The separated ACh and choline reacted to give a stoichiometric yield of hydrogen peroxide, which was electrochemically detected at a platinum electrode at a potential of +500 mV versus an Ag/AgCl reference electrode (Antek, Leiden, The Netherlands). The mobile phase, 0.2 M aqueous potassium phosphate buffer pH 8.0, containing 1 mM tetramethylammonium hydroxide, was delivered by a pump (Shimadzu LC-10AD) at 0.35–0.45 ml/min. The detection limit of the assay was ≈10 fmol/injection. The time required to complete a chromatogram was 4–5 min.
At the end of the dialysis experiments, placements of cannulae were checked histologically. Only the animals with correctly placed probes were included in the statistical analysis.
Statistical Analysis.
PREG S values had a log normal distribution, and a logarithmic transformation was applied to the data for statistical analysis. Microdialysate outputs in each animal were calculated as a percent of baseline, 100% baseline being the average of the last six pre-drug concentrations (fmol/min).
ANOVA was used to compare the scores among the groups for one variable (two-way ANOVA, treatment effect) and the time course of repeated behavioral measures among the groups (two-way ANOVA, interaction treatment × time). This was followed by post hoc comparison using the Newman-Keuls test. The Pearson’s correlation was used to compare PREG S levels and the memory performance and the Spearman’s correlation was employed to compare the memory performances in the water maze and Y-maze. The effect of i.p. PREG S injection on hippocampal PREG S content was analyzed using the Mann–Whitney U test. Student’s t test was used to analyze the effects of PREG S injections on memory performance in the Y-maze and also to compare the percentage values in the Y-maze to the random score of 33%.
Experimental Design.
Five experiments were performed in the following order: (i) In the first experiment, the concentrations of PREG S were measured in the hippocampus, amygdala, striatum, prefrontal cortex, and parietal cortex of single aged (24-month-old) and adult (3-month-old) male rats. In this experiment, the memory performance of aged rats was investigated in both the Morris water maze and the Y-maze. (ii) In the second experiment, the hippocampal content of PREG S was measured in 22-month-old aged rats (n = 22) 1 and 7 h after intraperitoneal injection of vehicle or PREG S. (iii) In the third and fourth experiments, the effects of either intraperitoneal or intrahippocampic PREG S injection on the memory performance of deficient aged rats were evaluated. (iv) In the fifth experiment, the effects of PREG S on extracellular concentrations of ACh in the hippocampus were assessed in adult rats (3-month-old).
RESULTS
PREG S Levels in Hippocampus.
Since the rats had to be killed at different day times, due to the protocol of behavioral tests, a preliminary experiment verified that brain PREG S does not undergo circadian variations. Concentrations measured every 4 h starting 2 h after lights on were 12.6 ± 1.7, 13.1 ± 2.4, 12.0 ± 2.6, 12.4 ± 2.4, 11.9 ± 2.5, and 10.8 ± 1.5 ng/g, respectively (mean SD, n = 7–10).
Hippocampal PREG S levels followed a log normal distribution [Shapiro–Wilks test, young rats (n = 12): w = 0.92, not significant (ns); old rats (n = 28): w = 0.93, ns], hence a logarithmic transformation was applied to the data for statistical analysis. Significantly lower levels were found in aged rats (Fig. 1), although the values were highly scattered and reached, in about half the animals, the same range as those of young ones (adult rats, 1.19 ± 0.16 log ng/g tissue, mean ± SD; aged rats, 0.98 ± 0.25 log ng/g; Student’s t test, t = 2.76, df 39, P < 0.001). The sensitivity of steroid RIA precluded more precise anatomical resolution.
Figure 1.
Individual PREG S levels (log ng/g tissue) in the hippocampus of young adult (3 month old, n = 12) and aged (24 month old, n = 28) rats. Horizontal lines represent the median of each distribution. A marked variability was observed in the aged rats and their median score was below the lowest young adult score.
Hippocampal PREG S and Cognitive Performance.
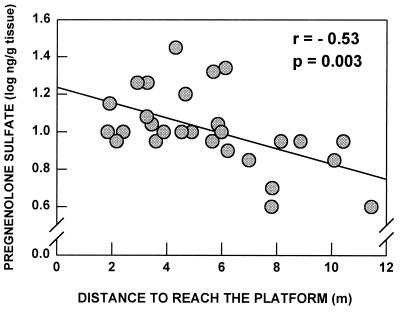
Individual hippocampal PREG S levels were correlated with the cognitive performance of each aged animal tested previously in the water maze (Fig. 2): not only a low level of PREG S was associated with a low memory performance measured during the last four days of the test (days 10–13), as indicated by the longer distance covered before reaching the submerged platform, but also PREG S and distance were linked by a significant negative correlation [y = (−7.01 ± 2.18)x + 12.61, r = −0.53, p = 0.003]. Moreover, the concentrations of PREG S were measured in other brain areas (data not shown). There was no relationship between the PREG S content in these areas and the performance in the water-maze (Pearson’s correlations: amygdala, r = −0.10, ns; prefrontal cortex, r = 0.08, ns; parietal cortex, r = −0.20, ns; striatum, r = 0.25, ns). All aged rats behaved similarly in the control procedure with the visible platform and they exhibited neither visual nor motor deficit.
Figure 2.
Learning performance of aged rats in the water-maze: correlation with PREG S levels in the hippocampus. PREG S concentrations were expressed in log (ng/g). The performance was expressed as the mean distance to reach the hidden platform during the last four days of the test. Low PREG S levels were linked with longer distances, i.e., worse performances [y = (−7.01 ± 2.18)x + 12.61].
The memory performance of the same aged rats was also assessed in the Y-maze after a 6 h ITI, and was compared with the one previously measured in the water maze: the rats that explored preferentially the novel arm of the Y-maze had covered a shorter distance in the water maze than those who performed at chance level (Spearman’s rank test, ρ 0.67, P < 0.001). None of the rats exhibited any deficit of the spontaneous exploration of the novel arm.
Thus, converging results in both tests likely reflect genuine differences in memory performance.
Correction of Memory Deficit After Injection of PREG S.
We assessed the correction of memory performance in the Y-maze by a single injection of PREG S, with the additional advantage to limit the extent of steroid metabolism (thanks to the shorter time interval between injection and sacrifice). In the second experiment, the hippocampal content of PREG S was measured in 22-month-old rats 1 and 7 h after intraperitoneal injection of vehicle or PREG S (47.5 mg/4 ml per kg). In the vehicle injected rats, the hippocampal concentrations of PREG S were similar at 1 and 7 h intervals and were pooled (16.6 ± 1.6 ng/g, mean ± SEM, n = 10). In PREG S treated rats, the hippocampal content of PREG S was increased to 77.7 ± 15.2 ng/g at 1 h (n = 12) and to 117.5 ± 28.9 ng/g at 7 h (n = 12) (Mann–Whitney test, P < 0.01 vs. vehicle injected controls). The plasma concentrations of PREG S were also measured. They were 3.5 ± 0.4 ng/ml in vehicle injected rats, and were increased to 4412 ± 367 ng/ml at 1 h and 1045 ± 288 ng/ml at 7 h. The large difference between plasma and hippocampal values is in accordance with the fact that steroid sulfates hardly cross the blood-brain barrier. Interestingly, PREG S concentration continued to increase in hippocampus at 7 h despite a marked decline in plasma.
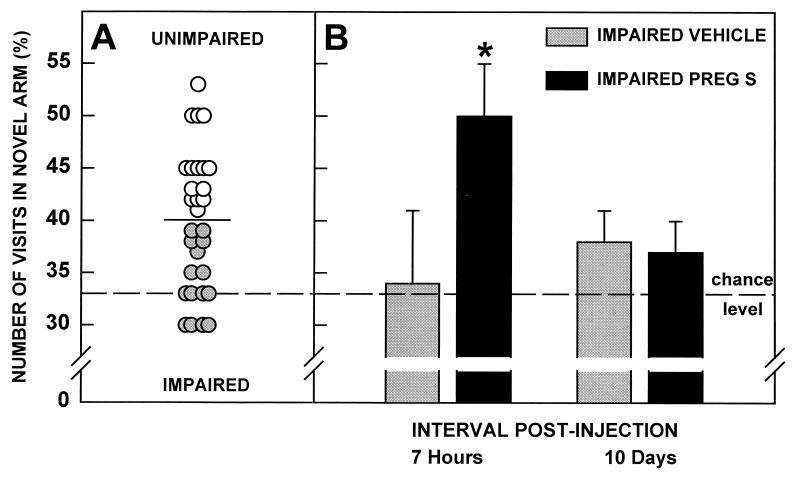
Thereafter, in the third experiment, rats were tested for their memory performance in the Y-maze (ITI = 6 h), and were split into two equal sub-groups with respect to the median score (Fig. 3A). As previously observed with the group of aged rats of the first experiment, the performance of the subgroup below median score did not differ from chance level. One week later, seven rats received PREG S intraperitoneally, whereas the eight others only received vehicle. One hour later, each rat underwent an acquisition trial in the Y-maze, and a retention trial 6 h later, i.e., 7 h after injection. Ten days later, the animals were retested in the Y-maze (ITI = 6 h). The Y-maze test was performed by an investigator unaware of the previous treatments of the animals. Seven hours postinjection the subgroup of impaired animals treated with PREG S had a higher performance than that observed prior to the injection (Newman Keuls, P < 0.05) (Fig. 3B). This subgroup also performed better than the vehicle subgroup of impaired animals (Newman-Keuls, P < 0.01) and chance (Student’s t test, t = 3.34, df = 6, P < 0.02). This difference was not accounted for by differences in motivation or activity between groups: both groups behaved in a similar way in the control task (ITI = 1 min: vehicle subgroup: 49 ± 4%, mean ± SEM; treated subgroup: 52 ± 4%; Student’s t test, t = 0.67, df = 13; ns), and made a similar number of visits to the three arms of the Y-maze during the first 2 min of the retention trial (vehicle subgroup: 4.3 ± 0.3, mean ± SEM; treated subgroup: 4.7 ± 0.4; Student’s t test, t = 0.54, df = 13; ns). In contrast, the performance of the subgroup treated with vehicle alone did not differ from chance (Student’s t test, t = 0.6, df = 7, ns). Ten days later, the performances of both subgroups returned to chance level (PREG S, Student’s t test, t = 1.33, df = 6, ns; vehicle, Student’s t test, t = 1.66, df = 7, ns).
Figure 3.
Effects of intraperitoneal PREG S injection on the performance of impaired aged rats in the Y-maze. (A) Male rats, 23 months old (n = 30) were tested for their memory performance in the Y-maze (ITI = 6 h). The parameter measured was the number of visits in novel arm (%). The horizontal bar represents the median of the distribution. Rats were divided in two groups: impaired rats ( ) (n = 15), whose score was below the median value; unimpaired rats (○) (n = 15), whose score was above median. The mean value of the impaired group did not differ from chance level (t = 1.0, df = 14, ns), while the mean value of the unimpaired group differed from chance level (t = 6.0, df = 14, ns). (B) The impaired animals (n = 15) were split into two subgroups. Vehicle injection had no effect on the performances of impaired rats (n = 8) in the Y-maze (ITI = 6 h). In contrast, the subgroup treated with PREG S (n = 7) performed significantly above chance level 7 h postinjection (t = 5.5, df = 6; ∗, P < 0.02), but returned to chance level 10 days later (t = 2.0, df = 6, ns).
In the fourth experiment, animals exhibited a similar memory performance in the Y-maze (ITI = 6 h), before and 10 days after the implantation of chronic indwelling cannulae (surgery effect, F(1, 23) = 3.8, ns). They were split into two subgroups: impaired (number of visits in novel arm 28 ± 2%, mean ± SEM, n = 12) and unimpaired rats (50 ± 2%, mean ± SEM, n = 12; Student’s t test, t = 8.7, df = 22; P < 0.001). One week later, impaired animals were retested in the Y-maze (ITI = 6 h). Immediately after the acquisition trial, half of them received bilateral infusions of PREG S (5 ng, n = 6) and the other half isotonic saline (n = 6) directly into the dorsal hippocampus. The bilateral infusion of PREG S into the dorsal hippocampus markedly improved the memory performance of impaired animals 6 h later (treatment effect, F(1, 5) = 19.7, P < 0.01) (Fig. 4). For this time-interval, animals treated with PREG S performed better than chance level (Student’s t test: t = 4.1, df = 5, P < 0.01) and better than the vehicle injected group (t = 3.1, df = 10, P < 0.01), the performance of which did not differ from chance (Student’s t test, t = 0.2, df = 5, ns). It was again verified that the effect of PREG S was not related to a change of motivation or activity because the number of visits of the novel arm was similar for both subgroups in the (ITI = 1 min) control test (vehicle group: 55 ± 5%, mean ± SEM; treated group: 47 ± 2%; Student’s t test, t = 1.4, df = 10, ns). Seven days after the injection, both groups exhibited their preinjection performance, i.e., animals that received PREG S returned to chance level (PREG S, Student’s t test, t = 1.1, df = 5 ns; vehicle, Student’s t test, t = 0.3, df = 5, ns).
Figure 4.
Effects of PREG S bilateral infusion into the hippocampus on the performance of impaired aged rats in the Y-maze. Impaired male rats, 22 months old (n = 12) were split into two subgroups and received infusion of PREG S or vehicle. Vehicle injection had no effect on the performances of impaired rats (n = 6) in the Y-maze (ITI = 6 h). In contrast, the subgroup treated with PREG S (n = 6) performed significantly above chance level 6 h postinjection (t = 4.1, df = 5, ∗∗, P < 0.01), but returned to chance level 7 days later (t = 1.1, df = 5, ns).
Increase of Extracellular Concentrations of ACh in the Hippocampus After Injection of PREG S.
Two groups of adult rats were submitted to delayed i.c.v. injection of PREG S (20 μg/20 μl per 4 min, n = 7) or vehicle (NaCl 0.9%, n = 6) during the microdialysis experiment. ACh levels of both groups were identical before injection. The averaged values of six baseline time-points were: vehicle group, 38 ± 5 fmol/min; PREG S group, 40 ± 10 fmol/min; Student’s t test, t = 0.34, df = 11, ns). Extracellular concentrations of ACh in the hippocampus increased with i.c.v. injection of PREG S (20 μg) [treatment effect, F(1, 11) = 11.9; P < 0.001] (Fig. 5). ACh concentrations, expressed as the percentage of baseline, were significantly larger 20, 30, and 40 min after the injection of PREG S than after vehicle (Newman-Keuls, P < 0.001, P < 0.01, and P < 0.001, respectively).
Figure 5.
Effects of the i.c.v. injection of PREG S (20 μg) on extracellular concentrations of ACh in the hippocampus. The output of ACh, expressed as the percentage of baseline, is increased 20, 30, and 40 min postinjection in PREG S group compared with vehicle group (∗∗, P < 0.01, ∗∗∗, P < 0.001).
DISCUSSION
Whereas several cross-sectional studies have documented the progressive decline of dehydroepiandrosterone sulfate (DHEA S) in human blood with age (reviewed in ref. 30), studies of neurosteroids in aging mammalian brain are still lacking. The present study describes for the first time a significant and selective decrease of hippocampal PREG S concentrations in 24-month-old male rats compared with young (3-month-old) adults. Moreover, the individual values in aged rats were widely scattered and were in the same range as in young ones in about half the aged rat population. More importantly, the interindividual differences observed in the spatial memory performances of the aged rats were positively correlated with the hippocampal concentration of PREG S. This is the first demonstration of a physiological relationship between endogenous neurosteroid levels and cognitive performance. The i.p. and also the intrahippocampic injection of PREG S could temporarily correct the memory deficit of aged rats. Several previous reports have documented the possible memory enhancing properties of PREG S injected either peripherally (6, 31, 32), i.c.v. (4, 7), or into brain structures (5, 9). However, in the majority of previous studies, the training of animals included punishment or reward and their significance in terms of memory performance has been questioned (12). We have used two different spatial memory tasks, one of them included a low emotional demand and the correlation of performances in both tests was strongly indicative of genuine memory appraisal. No previous report on the memory-enhancing properties of PREG S has checked the actual increase of PREG S in brain and particularly in the hippocampus. The present work extends a previous report showing that exogenous DHEA or DHEA S can improve memory in aged mice (33). However, we are the first to document the correction of a spontaneous memory deficit by the compensation of decreased endogenous neurosteroid levels. Finally, the intrahippocampal injection of PREG S was done immediately after the acquisition trial, confirming that the neurosteroid selectively reinforces memory retention (4, 5).
The mechanisms underlying the memory enhancing activity of PREG S are presently unknown. After injection of PREG S, the concentrations of its metabolites PREG, progesterone, 5α-pregnane-3,20-dione, and 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) are increased in the rat brain (13). DHEA is also a potential metabolite of PREG, even the biochemical steps of its formation in brain are still debated (34). The time elapsed between injection of PREG S and the observed effect on memory would be sufficient for a mechanism involving any of its metabolites. This point, although unlikely, merits further investigation with the use of appropriate metabolic inhibitors. Alternatively, a specific PREG S binding component in synaptosomal membranes has been reported (34). This component might be a receptor proper, although its transduction mechanisms are unknown. PREG S is both a γ-aminobutyric acid (GABA) antagonist at GABAA receptors (35) and a positive allosteric modulator at the N-methyl-d-aspartate receptor (36). Based on the present results, it could be hypothesized that the neuromodulatory pathways of PREG S may reinforce neurotransmitter system(s) that decline with age. Central cholinergic neurotransmission, that is in turn modulated by GABAergic and glutamatergic afferents (37, 38), represents a plausible candidate for these steroid effects. DHEA S, which has neuropharmaceutical properties close to those of PREG S, might play in the human the role subserved by PREG S in rodents.
Indeed, ACh systems in the basal forebrain, thought to be involved in the regulation of memory processes, are altered in normal aging, and degenerative changes in cholinergic nuclei correlate with memory impairment in aged rats (22). We have previously demonstrated that a local infusion of PREG S into the nucleus basalis magnocellularis, the main source of the cortical cholinergic innervation, enhanced the mnemonic performance in young rats (5). An injection of PREG S has been also shown to reverse the amnestic effects of the muscarinic cholinergic receptor blocker, scopolamine in mice (3, 11). Finally, we demonstrate in the present study that i.c.v. injection of PREG S stimulates ACh release in the adult rat hippocampus, in accordance with previous reports showing that DHEA S, which has GABAergic effects almost identical to those of PREG S, enhances hippocampal ACh release (39), whereas allopregnanolone, which has effects on GABAA receptors opposite to those of PREG S, has an inhibitory effect (5, 40).
In conclusion, our results suggest that hippocampal content of PREG S plays a physiological role in preserving and/or enhancing cognitive abilities in aged animals, possibly via an interaction with central cholinergic systems, and that neurosteroids should be studied in the context of prevention and/or treatment of age-related memory disorders, including neurodegenerative pathologies like Alzheimer disease in which ACh neurotransmission is predominantly affected (41).
Acknowledgments
We thank J. M. Claustrat, M. C. Donat, M. Kharouby, and C. Legris for technical or editorial assistance. Supported by Institut National de la Santé de la Recherche Médicale, the Mathers Foundation, the Université de Bordeaux II, and the Conseil Régional d’Aquitaine.
ABBREVIATIONS
- PREG S
pregnenolone sulfate
- DHEA S
dehydroepiandrosterone sulfate
- ACh
acetylcholine
- i.c.v.
intracerebroventricular
- GABA
γ-aminobutyric acid
- ITI
intertrial interval
- A
anteroposteriorly
- L
laterally
- V
ventrally
References
- 1.Schumacher M, Robel P, Baulieu E E. Dev Neurosci. 1996;18:6–21. doi: 10.1159/000111391. [DOI] [PubMed] [Google Scholar]
- 2.Bitran D, Purdy R H, Kellogg C K. Pharmacol Biochem Behav. 1993;45:423–428. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- 3.Roberts E. In: The Biological Role of Dehydroepiandrosterone (DHEA) Kalimi M, Regelson W, editors. Berlin: de Gruyter; 1990. pp. 13–42. [Google Scholar]
- 4.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayo W, Dellu F, Robel P, Cherkaoui J, Le Moal M, Baulieu E E, Simon H. Brain Res. 1993;607:324–328. doi: 10.1016/0006-8993(93)91524-v. [DOI] [PubMed] [Google Scholar]
- 6.Isaacson R L, Varner J A, Baars J-M, de Wied D. Brain Res. 1995;689:79–84. doi: 10.1016/0006-8993(95)00493-a. [DOI] [PubMed] [Google Scholar]
- 7.Mathis C, Paul S M, Crawley J N. Psychopharmacology. 1994;116:201–206. doi: 10.1007/BF02245063. [DOI] [PubMed] [Google Scholar]
- 8.Cheney D L, Uzunov D, Guidotti A. NeuroReport. 1995;6:1697–1700. doi: 10.1097/00001756-199508000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Flood J F, Morley J E, Roberts E. Proc Natl Acad Sci USA. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melchior C L, Ritzmann R F. Pharmacol Biochem Behav. 1996;53:51–56. doi: 10.1016/0091-3057(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 11.Meziane H, Mathis C, Paul S M, Ungerer A. Psychopharmacology. 1996;126:323–330. doi: 10.1007/BF02247383. [DOI] [PubMed] [Google Scholar]
- 12.Porsolt R G, Roux S, Wettstein J G. Drug Dev Res. 1995;35:214–229. [Google Scholar]
- 13.Romeo E, Cheney D L, Zivkovic I, Costa E, Guidotti A. J Pharmacol Exp Ther. 1994;270:89–96. [PubMed] [Google Scholar]
- 14.Warner M, Gustafsson J-A. Front Neuroendocr. 1995;126:224–236. doi: 10.1006/frne.1995.1008. [DOI] [PubMed] [Google Scholar]
- 15.Koenig H L, Schumacher M, Ferzaz B, Do Thi A N, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu E E. Science. 1995;268:1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- 16.Rapp P R, Amaral D G. Trends Neurosci. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- 17.Landfield P W. Neurobiol Aging. 1988;9:571–579. doi: 10.1016/s0197-4580(88)80116-7. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H, Otto T. Behav Neural Biol. 1992;57:2–36. doi: 10.1016/0163-1047(92)90724-i. [DOI] [PubMed] [Google Scholar]
- 19.Corpéchot C, Young J, Calvel M, Wehrey C, Veltz J N, Touyer G, Mouren M, Prasad V V K, Banner C, Sjövall J, Baulieu E E, Robel P. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- 20.Morris R. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. Brain Res. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong D M, Sheffield R, Buszaki G, Chen K S, Hersh L B, Nearing B, Gage F H. Neurobiol Aging. 1993;14:457–470. doi: 10.1016/0197-4580(93)90104-j. [DOI] [PubMed] [Google Scholar]
- 23.Stewart C A, Morris R G M. In: Behavioral Neuroscience, A Practical Approach. Sahgal A, editor. New York: Oxford Univ. Press; 1993. pp. 107–122. [Google Scholar]
- 24.Conrad C D, Galea L A, Kuroda Y, McEwen B S. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 26.Damsma G, Westerink B H C. In: Microdialysis in the Neurosciences. Robinson T E, Justice J, editors. Amsterdam: Elsevier; 1991. pp. 237–252. [Google Scholar]
- 27.Day J C, Fibiger H C. J Neurochem. 1994;63:2086–2092. doi: 10.1046/j.1471-4159.1994.63062086.x. [DOI] [PubMed] [Google Scholar]
- 28.Day J C, Piazza P V, Le Moal M, Maccari S. Eur J Neurosci. 1997;9:1130–1136. doi: 10.1111/j.1460-9568.1997.tb01466.x. [DOI] [PubMed] [Google Scholar]
- 29.Damsma G, Lammerts D, van BuerenWesterink B H C, Horn A S. Chromatographia. 1987;24:827–831. [Google Scholar]
- 30.Berr C, Lafont S, Debuire B, Dartigues J-F, Baulieu E-E. Proc Natl Acad Sci USA. 1996;93:13410–13415. doi: 10.1073/pnas.93.23.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isaacson R L, Yoder P E, Varner J. Behav Neural Biol. 1994;61:170–176. doi: 10.1016/s0163-1047(05)80071-8. [DOI] [PubMed] [Google Scholar]
- 32.Frye C A, Sturgis J D. Neurobiol Learn Mem. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- 33.Flood F, Smith G E, Roberts E. Brain Res. 1988;447:269–278. doi: 10.1016/0006-8993(88)91129-8. [DOI] [PubMed] [Google Scholar]
- 34.Robel P, Baulieu E E. Trends Endocrinol Metab. 1994;5:1–8. doi: 10.1016/1043-2760(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 35.Majewska M D. Prog Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 36.Wu F S, Gibbs T T, Farb D H. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- 37.Giovannini M G, Camilli F, Mundula A, Pepeu G. Neurochem Int. 1994;25:23–26. doi: 10.1016/0197-0186(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 38.Chang H T, Tian Q, Herron P. Neuroscience. 1995;68:207–220. doi: 10.1016/0306-4522(95)00109-v. [DOI] [PubMed] [Google Scholar]
- 39.Rhodes M E, Li P-K, Flood J F, Johnson D A. Brain Res. 1996;733:284–286. doi: 10.1016/0006-8993(96)00751-2. [DOI] [PubMed] [Google Scholar]
- 40.Dazzi L, Sanna A, Cagetti E, Concas A, Biggio G. Brain Res. 1996;710:275–280. doi: 10.1016/0006-8993(95)01478-0. [DOI] [PubMed] [Google Scholar]
- 41.Coyle J T, Price D L, DeLong M R. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]