Abstract
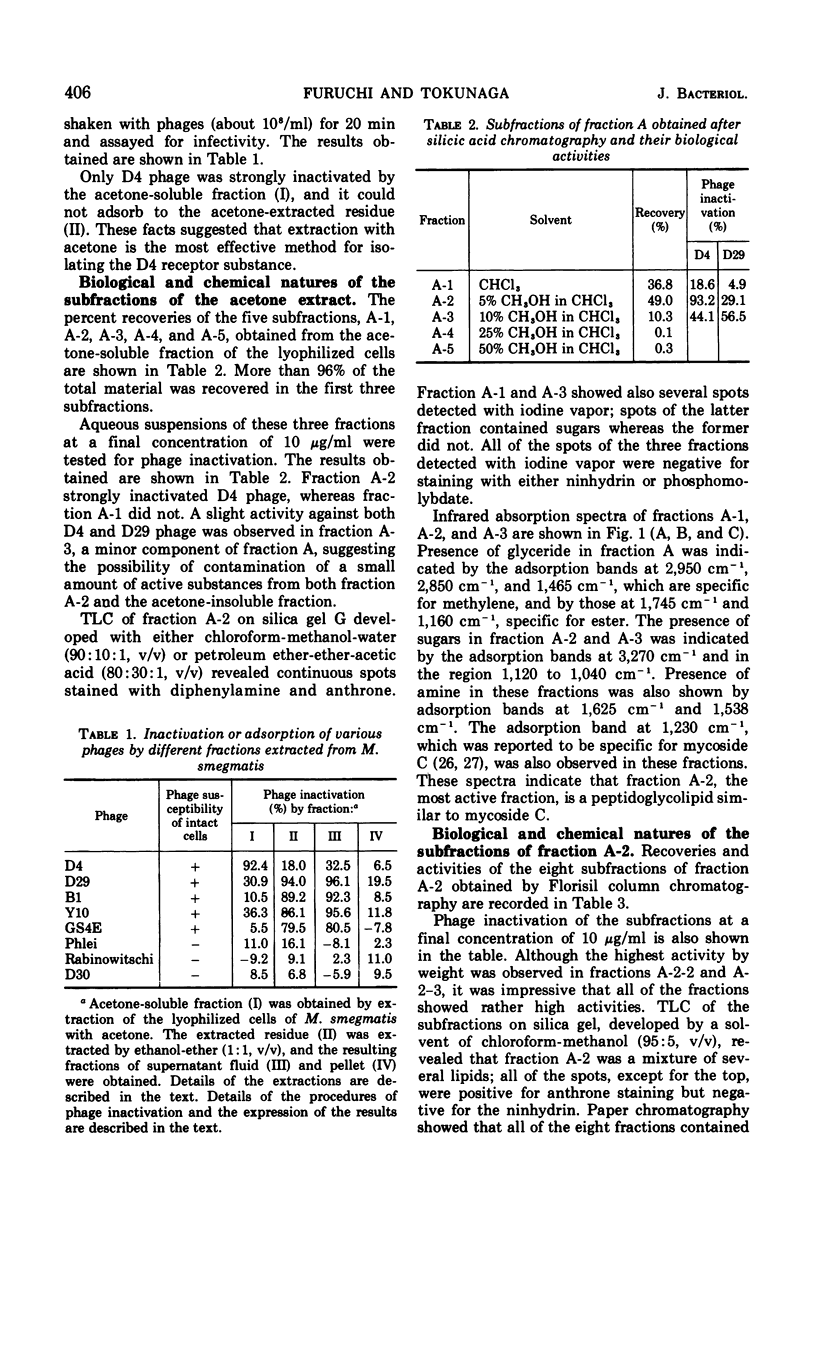
The acetone-soluble fraction extracted from lyophilized cells of Mycobacterium smegmatis ATCC 607 inhibited D4, a species-specific phage active against M. smegmatis. Evidence is presented indicating that the D4 inhibition was caused by the phage receptor substance(s) contained in this fraction. A fraction eluted from silicic acid with chloroform-methanol (95:5, v/v) showed the strongest inhibition of D4 phage. This fraction contained sugars and amino acids, and its infrared absorption spectrum was practically identical with those of the mycoside C isolated from the other species of mycobacteria. Further fractionation revealed that the active material was a mixture of several closely related peptidoglycolipids all of which showed, more or less, the phage inhibition. One of the compounds was purified and partially characterized; it contains three different amino acids, allo-threonine, alanine, and phenylalanine, at a molar ratio of 1:1:1, and also three different deoxyhexoses, probably 6-deoxytalose, 3,4-di-o-methylrhamnose, and 2,3,4-tri-o-methylrhamnose. A tentative name of “mycoside Csm” is proposed for this substance which possesses a slightly different structure from the known types of mycoside C and is probably specific for the species of M. smegmatis. A fraction extracted from the D4-resistant mutant of M. smegmatis ATCC 607 by acetone and then by chloroform-methanol (95:5, v/v) showed no phage inhibition and had no sugar component. In addition, this fraction contained lysine, serine, and a small amount of both glycine and an unidentified amino acid.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON J. S. D., EDELMAN J. The carbohydrates of the Jerusalem artichoke and other Compositae. Biochem J. 1951 Jan;48(1):114–126. doi: 10.1042/bj0480114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPUT M., MICHEL G., LEDERER E. STRUCTURE DU MYCOSIDE C2 DE MYCOBACTERIUM AVIUM. Biochim Biophys Acta. 1963 Oct 29;78:329–341. doi: 10.1016/0006-3002(63)91643-3. [DOI] [PubMed] [Google Scholar]
- CHAPUT M., MICHEL G., LEDERER E. [On the mycoside Cm, a new peptido-glycolipid isolated from Mycobacterium marianum]. Experientia. 1961 Mar 15;17:107–108. doi: 10.1007/BF02160810. [DOI] [PubMed] [Google Scholar]
- CHAPUT M., MICHEL G., LEDERER E. [Structure of mycoside Cm, peptidoglycolipid of Mycobacterium marianum]. Biochim Biophys Acta. 1962 Sep 24;63:310–326. doi: 10.1016/0006-3002(62)90685-6. [DOI] [PubMed] [Google Scholar]
- Castelnuovo G., Bellezza G., Yamanaka S. Phage inhibiting substances of mycobacteria. I. The nature of phage inhibiting substances of Mycobacterium phlei. Ann Inst Pasteur (Paris) 1970 Sep;119(3):302–311. [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Drozdz B., Kowalewski Z. Selective detection of various types of sugars present in cardiac heterosides. J Chromatogr. 1965 Oct;20(1):188–188. doi: 10.1016/s0021-9673(01)97394-7. [DOI] [PubMed] [Google Scholar]
- FROMAN S., WILL D. W., BOGEN E. Bacteriophage active against virulent Mycobacterium tuberculosis. I. Isolation and activity. Am J Public Health Nations Health. 1954 Oct;44(10):1326–1333. doi: 10.2105/ajph.44.10.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Ionesco H., Schaeffer P. Teichoic acids as components of a specific phage receptor in Bacillus subtilis. Biochim Biophys Acta. 1966 Aug 24;124(2):415–417. doi: 10.1016/0304-4165(66)90211-x. [DOI] [PubMed] [Google Scholar]
- IKAWA M., SNELL E. E., LEDERER E. Occurrence of D-phenylalanine, D-allothreonine and other D-amino-acids in peptido-lipids of bacterial origin. Nature. 1960 Nov 12;188:558–560. doi: 10.1038/188558a0. [DOI] [PubMed] [Google Scholar]
- JOLLES P., BIGLER F., GENDRE T., LEDERER E. [On the chemical structure of "myoside C", a peptide-glycolipid from Mycobacterium avium]. Bull Soc Chim Biol (Paris) 1961;43:177–192. [PubMed] [Google Scholar]
- Lanéelle M. A., Lanéelle G., Bennet P., Asselineau J. Sur les lipides d'une souche non-photochromogène de mycobactérie. Bull Soc Chim Biol (Paris) 1965;47(11):2047–2067. [PubMed] [Google Scholar]
- Lindberg A. A., Hellerqvist C. G. Bacteriophage attachment sites, serological specificity, and chemical composition of the lipopolysaccharides of semirough and rough mutants of Salmonella typhimurium. J Bacteriol. 1971 Jan;105(1):57–64. doi: 10.1128/jb.105.1.57-64.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLENNAN A. P. The monosaccharide units in specific glycolipids of Mycobacterium avium. Biochem J. 1962 Mar;82:394–400. doi: 10.1042/bj0820394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael J. G. The surface antigens and phage receptors in Escherichia coli B. Proc Soc Exp Biol Med. 1968 Jun;128(2):434–438. doi: 10.3181/00379727-128-33031. [DOI] [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- SELLERS M. I., BAXTER W. L., RUNNALS H. R. Growth characteristics of mycobacteriophages D28 and D29. Can J Microbiol. 1962 Jun;8:389–399. doi: 10.1139/m62-051. [DOI] [PubMed] [Google Scholar]
- SELLES M. I., RUNNALS H. R. Mycobacteriophage. I. Physicochemical characterization. J Bacteriol. 1961 Mar;81:442–447. doi: 10.1128/jb.81.3.442-447.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH D. W., RANDALL H. M., MACLENNAN A. P., LEDERER E. Mycosides: a new class of type-specific glycolipids of Mycobacteria. Nature. 1960 Jun 11;186:887–888. doi: 10.1038/186887a0. [DOI] [PubMed] [Google Scholar]
- SMITH D. W., RANDALL H. M., MACLENNAN A. P., PUTNEY R. K., RAO S. V. Detection of specific lipids in mycobacteria by infrared spectroscopy. J Bacteriol. 1960 Feb;79:217–229. doi: 10.1128/jb.79.2.217-229.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOKUNAGA T., SELLERS M. INFECTION OF MYCOBACTERIUM SMEGMATIS WITH D29 PHAGE DNA. J Exp Med. 1964 Jan 1;119:139–149. doi: 10.1084/jem.119.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga T., Kataoka T., Suga K. Phage inactivation by an ethanol-ether extract of Mycobacterium smegmatis. Am Rev Respir Dis. 1970 Feb;101(2):309–313. doi: 10.1164/arrd.1970.101.2.309. [DOI] [PubMed] [Google Scholar]
- Tokunaga T., Kataoka T., Suga K. [Bacteriophage receptor of Mycobacteria. 1. Preliminary experiments on the phage-receptor of Mycobacterium smegmatis]. Igaku To Seibutsugaku. 1968 Dec 10;77(6):225–229. [PubMed] [Google Scholar]
- Tokunaga T., Maruyama Y., Murohashi T. [Further studies on the phage typing of rapidly growing Mycobacteria]. Nihon Saikingaku Zasshi. 1965 Sep;20(9):554–559. doi: 10.3412/jsb.20.554. [DOI] [PubMed] [Google Scholar]
- VILKAS E., MIQUEL A. M., LEDERER E. [On the isolation and structure of fortuitine, peptidolipid from Mycobacterium fortuitum]. Biochim Biophys Acta. 1963 Apr 23;70:217–218. doi: 10.1016/0006-3002(63)90745-5. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Brock T. D. Purification and properties of a bacteriophage receptor material from Streptococcus faecium. Biochim Biophys Acta. 1966 Jun 29;121(2):298–314. doi: 10.1016/0304-4165(66)90119-x. [DOI] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]
- WHITE A., KNIGHT V. Effect of tween 80 and serum on the interaction of mycobacteriophage D-29 with certain mycobacterial species. Am Rev Tuberc. 1958 Jan;77(1):134–145. doi: 10.1164/artpd.1958.77.1.134. [DOI] [PubMed] [Google Scholar]



